Method for determining apixaban cleaning residues by high performance liquid chromatography
A high-performance liquid chromatography and apixaban technology, which is applied in the field of high-performance liquid chromatography for the determination of apixaban cleaning residues, can solve the problems of irrelevant reports of trace residues, and achieve improved peak shape and effective detection of trace residues , Improve reliability, reduce the effect of detection limit
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Determination of Apixaban Cleaning Residue Method by High Performance Liquid Chromatography:
[0033] Instruments and reagents: LC-2010A high performance liquid chromatography (quaternary pump, degassing unit, UV detector, column oven, automatic sampler, system monitor, LCsolution) (Shimadzu Corporation of Japan); (Manufacturer: Jiangsu Baozhong Baoda Pharmaceutical Co., Ltd., acetonitrile, methanol (Merck chromatographically pure), other reagents are analytically pure, and water is self-made fresh redistilled water.
[0034] Chromatographic condition selection: chromatographic column: Inertsil ODS-3V 5μm 250×4.6mm; mobile phase A: 30mmol / L ammonium acetate aqueous solution, mobile phase B: acetonitrile, isocratic elution; detection wavelength: 280nm; calculation method: external standard method ;Flow rate: 1.0 mLmin -1 ; Sensitivity: 1.0 AUFS; Injection volume: 10 μL; Column temperature: 40°C.
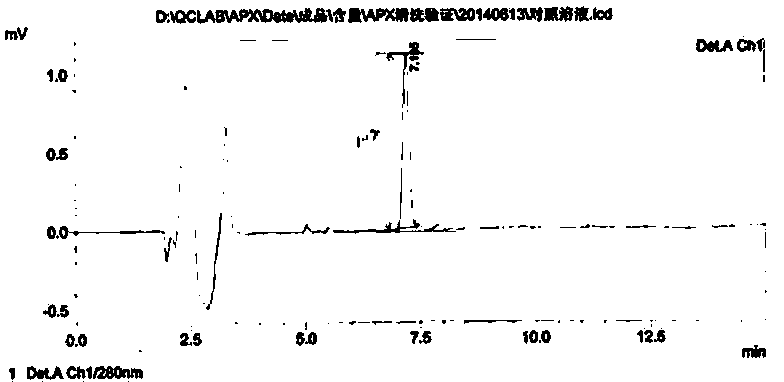
[0035] Specificity test: Methanol for rinsing of the sampling equipment,...
Embodiment 2
[0047] The precision test of the inventive method:
[0048] System precision: Take the control solution, inject 6 consecutive samples, and calculate the retention time and RSD of the peak area of apixaban. The RSD of the actual measured retention time is 0.03%; the RSD of the peak area is 0.59%
[0049] Method precision: 6 control solutions were prepared repeatedly, and each solution was injected once. The RSD of peak area / weighted sample volume of apixaban was 0.68%.
[0050] Intermediate precision: The method precision test was repeated by another analyst on another day on another instrument, and the RSD of the peak area / weighted sample volume of apixaban was 6.87%.
[0051] It shows that the reproducibility is good and the method is reliable.
Embodiment 3
[0053] The limit of detection test of the inventive method:
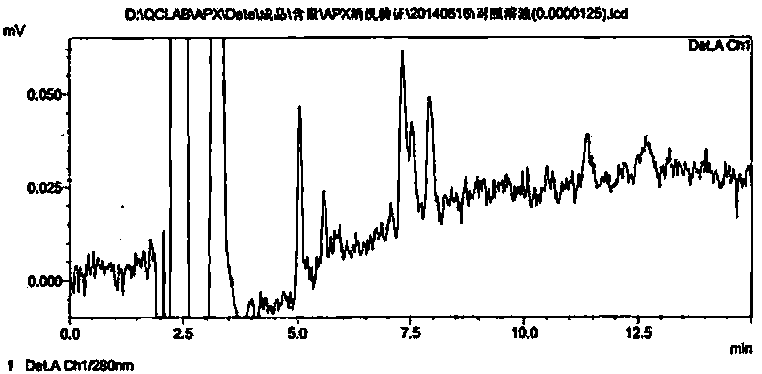
[0054] By injecting a low concentration of apixaban reference substance solution, when the signal-to-noise ratio (S / N) is 3, this concentration is its detection limit, and the detection limit solution is injected for 3 injections, and the RSD of the peak area is calculated and reported. Finally, the concentration of 0.0125ug / ml when S / N was determined to be about 2.8 was the limit of detection. The area RSD was 4.6%.
[0055]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com