A kind of recombinant immunodeficiency plasmid and virus and application
An immunodeficiency plasmid and immunodeficiency virus technology, which is applied to recombinant immunodeficiency plasmids and viruses and application fields, can solve the problems of inappropriate embedded genes and sites, single drug target, and inability to comprehensively evaluate the therapeutic effects of multiple drugs. , to achieve the effect of high-efficiency replication
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1: construct immunodeficiency plasmid of the present invention
[0047] Through upstream primers (nucleotide sequence shown in SEQ ID No.3) and downstream primers (nucleotide sequence shown in SEQ ID No.4) with Apa I and Nhe I restriction sites respectively, point mutation PCR Apa I and Nhe I restriction sites were respectively introduced at both ends of the nucleotide sequence shown in SEQ ID No.1, and then connected into the T vector;
[0048] F: ATCCTTTAGCGGGCCCCAAATCACTCTTTG (Apa I); R: CATGTGCTAGCCCTCATCCTGTCTACTTGCCACAC (Nhe I).
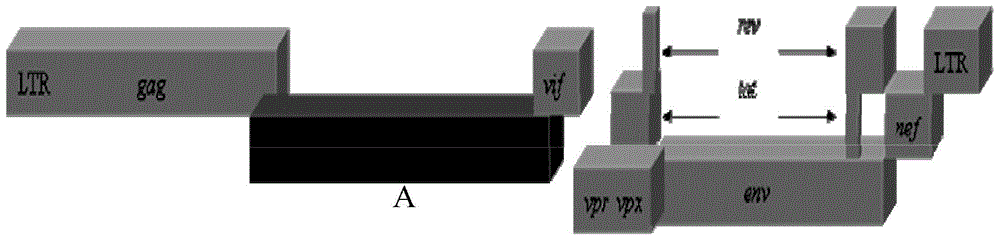
[0049] Using the half-length plasmid p239SpSp5' at the 5' end of SIVmac239 as a template, two pairs of primers 2982-F and 3736-R (nucleotide sequences shown in SEQ ID No.5-6), 3736 -F and 6307-R (nucleotide sequence shown in SEQ ID No.7-8) amplification, then use the obtained amplified product as template, through primer 2982-OV-F and 6307-OV-R (SEQ ID No. The nucleotide sequence shown in ID No.9-10) amplifies the 3539bpP-12...
Embodiment 2
[0059] Embodiment 2: the massive preparation of described immunodeficiency plasmid
[0060] The immunodeficiency plasmid was transformed into JM109 strain, and after plate culture at 30°C for 18-20 hours, a single colony was picked and inoculated into 5 mL of LB liquid medium containing ampicillin. After shaking at 30°C and 180rpm for 12 to 14 hours, take 1.5mL of bacterial culture solution for small plasmid extraction. After enzyme digestion and identification are correct, inoculate the remaining 3.5mL of bacterial culture solution into 1L of 2×YT liquid containing ampicillin medium. After shaking culture at 30°C and 180rpm for 12 hours, take 1.5mL of bacterial culture solution for small extraction again. After the enzyme digestion and identification are correct, refer to Qiagen’s instructions, and use Endofree Plasmid Giga Kit to prepare a large amount of plasmids from 1L of bacterial solution. . Proceed as follows:
[0061] (1) Centrifuge the bacterial solution at 4°C, 6...
Embodiment 3
[0071] Embodiment 3: the transfection packaging of immunodeficiency plasmid of the present invention
[0072]Use the immunodeficiency plasmid prepared in Example 2 to transfect 293T cells to obtain infectious complete virus particles, use a 6-well plate to culture 293T cells and obtain virus particles, the specific steps are as follows:
[0073] (1) Spread 293T cells into 6-well plates with 10% complete DMEM medium (5×10 5 / well), overnight culture, the cell density reached 70%, the purpose is to culture for a long time.
[0074] (2) Replace each well with 2ml of complete DMEM medium without antibiotics, and continue culturing for 2 hours.
[0075] (3) Dilute 5 μg of the immunodeficiency plasmid DNA in 650 μl of serum-free and antibiotic-free DMEM medium, and mix gently.
[0076] (4) Dilute 10 μl of Lipofectamine 2000 reagent (mix gently before use) in 650 μl of serum-free and antibiotic-free DMEM medium, and incubate at room temperature for 5 minutes.
[0077] (5) Mix the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com