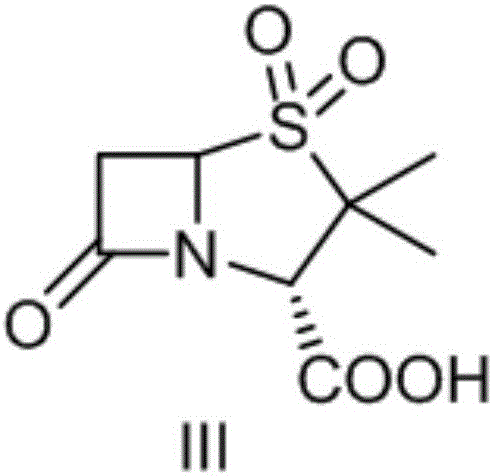
A kind of synthetic method of sulbactamic acid
A technology of sulbactamic acid and its synthetic method, which is applied in the field of sulbactamic acid synthesis, can solve the problems of cumbersome feeding operations, serious dust pollution, dark color, etc., and achieve the goals of improving reaction quality, avoiding dust pollution, and improving working environment Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
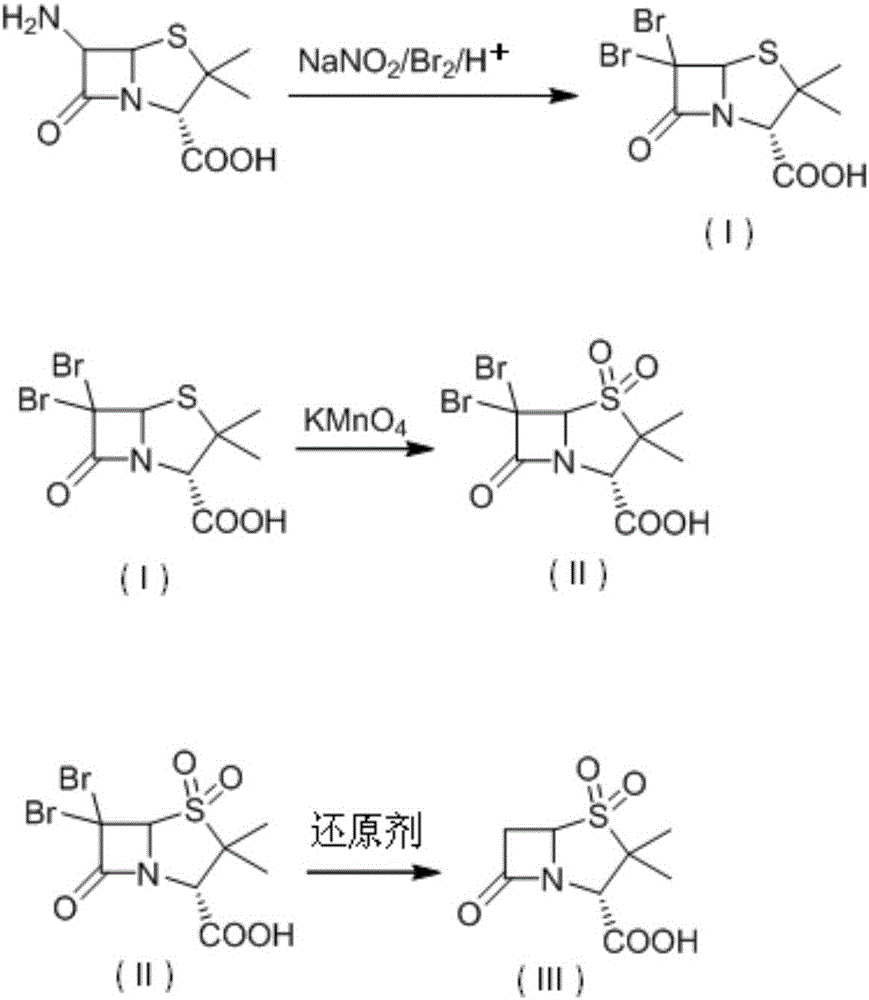
[0040] Add 200 mL of ethyl acetate into a four-necked flask, cool down to 0°C, add bromine (40.0 g, 0.235 mol), stir, and simultaneously add a saturated aqueous solution of sodium nitrite (12.5 g, 0.14 mol) and 6-aminopenicillium The acid solution of alkanoic acid (dissolve 20.0g of 6-aminopenicillanic acid in 20mL of 5% sulfuric acid solution) was added dropwise to the reaction solution at 0-5°C, and the drop was completed in about 1.0-1.5 hours. No trace was detected during the reaction. 6-aminopenicillanic acid breakdown products. After reacting at 10-15°C for 30 minutes, 1 mol / L sodium bisulfite solution was added dropwise until the reaction solution turned yellow, and the layers were separated after standing. The aqueous layer was extracted once with ethyl acetate (30 mL), and the organic phases were combined. The organic phase was extracted twice with 10% NaOH, and the organic phase was applied mechanically. After the combined aqueous phase HPLC detected that the compoun...
Embodiment 2
[0044] Add 220 mL of tetrahydrofuran into a four-necked flask, cool down to 0°C, add bromine (37.0 g, 0.231 mol), stir, and simultaneously add a saturated solution of sodium nitrite (12.5 g, 0.14 mol) and 6-aminopenicillanic acid Acid solution (20.0g of 6-aminopenicillanic acid dissolved in 15mL of 8% sulfuric acid solution), was added dropwise into the reaction solution at 0-5°C, and the drop was completed in about 1.0-1.5h. After reacting at 10-15°C for 30 minutes, 1 mol / L sodium bisulfite solution was added dropwise until the reaction solution turned yellow, and the layers were separated after standing. The aqueous layer was extracted once with tetrahydrofuran (30 mL), and the organic phases were combined. The organic phase was extracted twice with 10% NaOH, and the organic phase was used mechanically. After the combined aqueous phase HPLC detected that the compound I content was more than 99.0%, it was directly put into the next step reaction.
[0045] Add the aqueous solu...
Embodiment 3
[0048] Add 200 mL of ethyl acetate into a four-neck flask, cool down to 0°C, add bromine (48.0 g, 0.282 mol), stir, and simultaneously add a saturated aqueous solution of sodium nitrite (9.0 g, 0.101 mol) and 6-aminopenicillium The acid solution of alkanoic acid (dissolve 20.0g of 6-aminopenicillanic acid in 20mL of 15% hydrobromic acid aqueous solution) was added dropwise to the reaction solution at 0-5°C, and the drop was completed in about 1.0-1.5h. After reacting at 10-15°C for 30 minutes, 1 mol / L sodium bisulfite solution was added dropwise until the reaction solution turned yellow, and the layers were separated after standing. The aqueous layer was extracted once with ethyl acetate (30 mL), and the organic phases were combined. The organic phase was extracted twice with 10% NaOH, and the organic phase was used mechanically. After the combined aqueous phase HPLC detected that the compound I content was more than 99.0%, it was directly put into the next step reaction.
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com