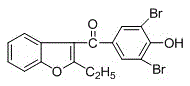
A kind of benzbromarone crystal form a and preparation method thereof
A technology of benzbromarone and crystal form, which is applied in the field of chemical pharmaceuticals, can solve the problems of poor industrial applicability of crystallization purification methods, poor stability of drug forms, and inestimable market potential, and achieve low cost, good crystal form stability, and conditions mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Preparation of benzbromarone crystal form A:
[0019] Take 27.5g of benzbromarone raw material and dissolve it in 90ml of acetone solvent under reflux condition at 56°C. After dissolving, the temperature is rapidly lowered, and solid is gradually precipitated. It is left to stand at 5°C for crystallization for 10 hours, filtered, and rinsed with a small amount of cold acetone. , the benzbromarone crystal form A can be obtained.
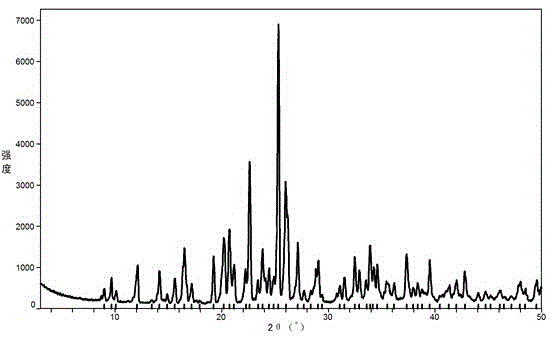
[0020] The powder X-ray diffraction pattern of the obtained benzbromarone crystal form A is as attached figure 2 shown.
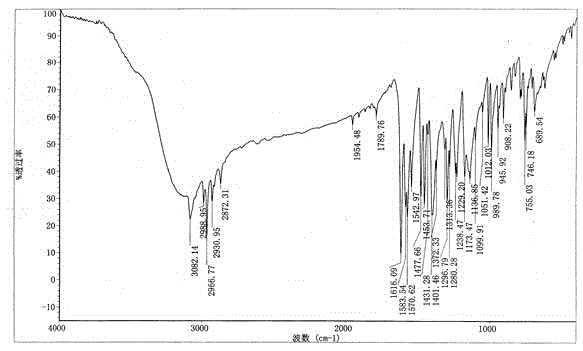
[0021] The infrared absorption spectrum of the obtained benzbromarone crystal form A is as follows image 3 shown.
[0022] The differential scanning calorimetry differential thermal analysis spectrum of the obtained benzbromarone crystal form A is attached Figure 4 shown.
[0023] The detection method and results of differential scanning calorimetry differential thermal analysis spectrum are as follows:
[0024] Benz...
Embodiment 2
[0032] Take 27.5g of benzbromarone raw material and dissolve it in 82ml of acetone solvent at 50°C. After dissolving, the temperature is rapidly lowered, and a solid is gradually precipitated. It is left to stand at 0°C for crystallization for 2 hours, filtered, and rinsed with a small amount of cold acetone. The benzbromarone crystal form A can be obtained.
Embodiment 3
[0034] Take 27.5g of benzbromarone raw material and dissolve it in 138ml of acetone solvent at 40°C. After dissolving, the temperature is rapidly lowered, and a solid is gradually precipitated. It is left to stand at 20°C for crystallization for 15 hours, filtered, and rinsed with a small amount of cold acetone. The benzbromarone crystal form A can be obtained.
[0035] Its related substance content of the benzbromarone crystal form A obtained by the preparation method of the present invention is far less than the standard of European Pharmacopoeia 7.0 edition (1465-1466 pages), and the content of related substances is shown in Table 1.
[0036] Table 1 Comparison of benzbromarone raw material and crystal form A sample related substances with European Pharmacopoeia standards
[0037]
[0038] The solid state of benzbromarone crystal form A obtained by this preparation method has the following conditions of color and related substances after 10 days, 20 days and 30 days unde...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com