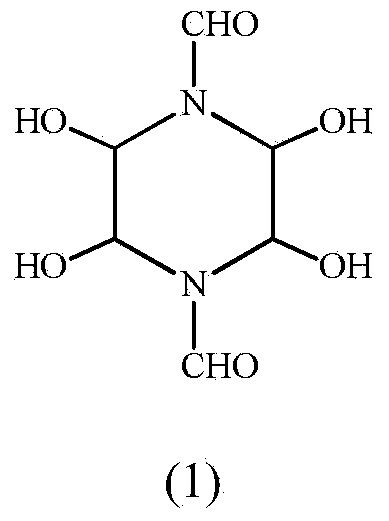
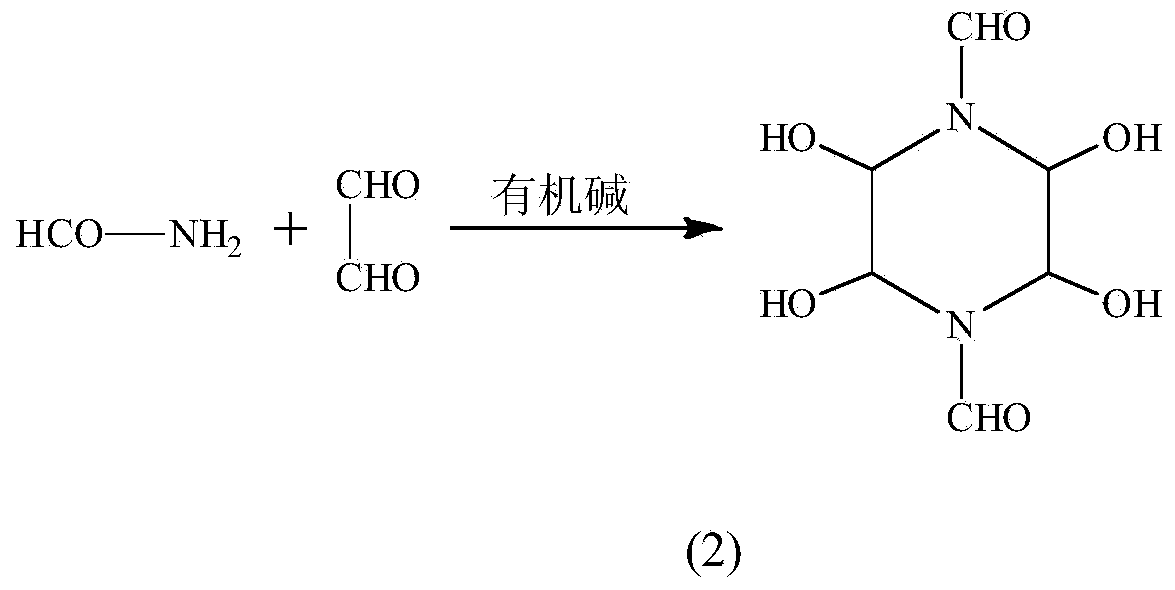
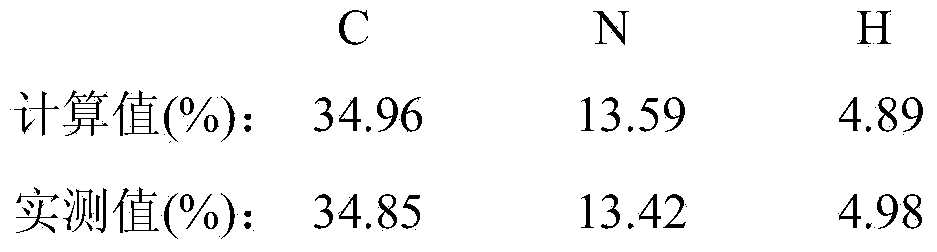Synthetic method of 1,4-diformyl-2,3,5,6-tetrahydroxypiperazine
A technology of tetrahydroxypiperazine and diformyl, which is applied in the synthesis field of piperazine compounds, can solve the problems of long synthesis period, product loss, and product purity can only reach 98%, so as to shorten the synthesis period and simplify the operation process , save the effect of the refining process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Add 145g 40% (1.0mol) glyoxal aqueous solution and 45g (1.0mol) formamide to a 500ml three-neck round bottom flask, stir to make it fully mixed, then add 10ml (0.075mol) triethylamine dropwise, control the temperature At 45°C, after 8 minutes of dropwise addition, a white precipitate appeared immediately, filtered while hot, washed with water and ethanol, and dried to obtain 82.5 g of a white powdery solid with a purity of 99.5% and a yield of 80%.
[0016] Melting point: 192~195°C (reported value 196°C).
[0017] Structure Identification:
[0018] 1 HNMR (DMSO-d 6 , δ / ppm): δ8.26(s, 2H), δ6.04(s, 2H), δ5.92(s, 2H), δ5.46(s, 2H), δ4.98(s, 2H) .
[0019] 13 CNMR (DMSO-d 6 , δ / ppm), δ: 164.4, 164.3, 79.3, 78.9, 72.5, 72.2.
[0020] Elemental analysis (C 6 h 10 N 2 o 6 ):
[0021]
[0022] Mass Spectrum: MS(m / e)206(M + ).
[0023] The above structural identification data prove that the substance obtained by this synthesis method is indeed 1,4-diformyl-2,3,...
Embodiment 2
[0025] Add 145g 40% (1.0mol) glyoxal aqueous solution and 45g (1.0mol) formamide to a 500ml three-neck round bottom flask, stir to make it fully mixed, then add 12ml (0.09mol) triethylamine dropwise, control the temperature At 45°C, after 10 minutes of dropwise addition, a white precipitate appeared immediately, which was filtered while hot, washed with water and ethanol, and dried to obtain 78.2 g of a white powdery solid with a purity of 99% and a yield of 75.9%.
Embodiment 3
[0027] Add 145g 40% (1.0mol) glyoxal aqueous solution and 45g (1.0mol) formamide to a 500ml three-neck round bottom flask, stir to make it fully mixed, then add 8ml (0.06mol) triethylamine dropwise, and control the temperature At 45°C, after 5 minutes of dropwise addition, a white precipitate appeared immediately, which was filtered while hot, washed with water and ethanol, and dried to obtain 75.2 g of a white powdery solid with a purity of 99.2% and a yield of 73%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com