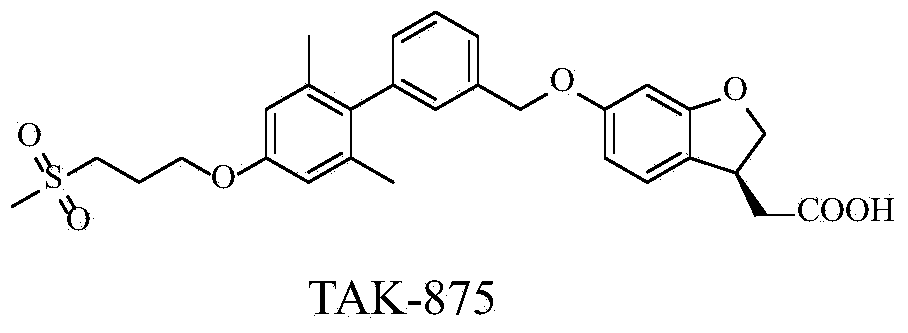
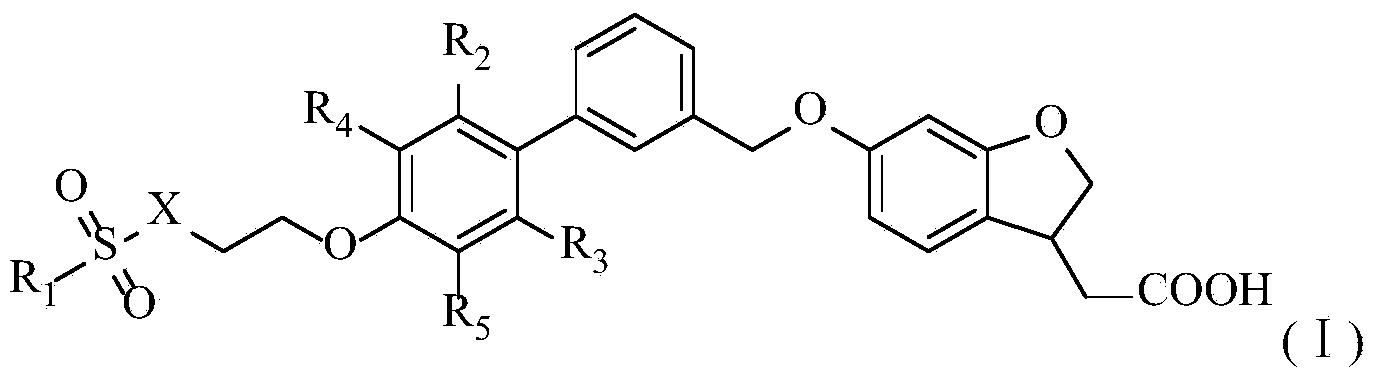
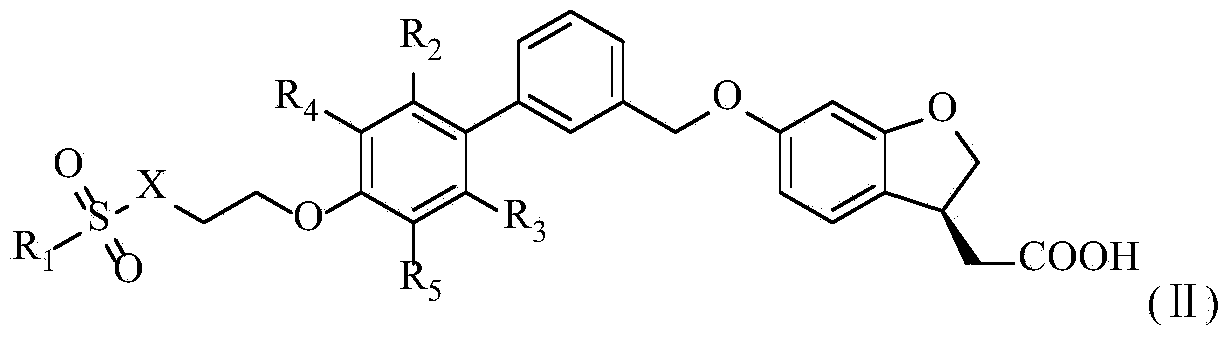
Aromatic polycyclic carboxylic acid derivative
A kind of medicine and compound technology, applied in the field of aromatic polycyclic carboxylic acid derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0283] Example 12-(6-((2',6'-dimethyl-4'-(2-(methylsulfonylamino)ethoxy)-[1,1'-biphenyl]-3-yl )methoxy)-2,3-dihydrobenzofuran-3-yl)acetic acid synthesis (preparation of compound 1)
[0284]
[0285] (1) Preparation of 4-(chloromethyl)-7-hydroxyl-2H-benzopyran-2-one
[0286]
[0287] Resorcinol (27.5g, 250mmol) was dissolved in acetic acid (60mL) and heated to 50°C for later use, ethyl 4-chloroacetoacetate (20.5g, 125mmol) was dissolved in acetic acid (20mL) and cooled in an ice-water bath, and concentrated sulfuric acid was slowly added (10mL), then add the acetic acid solution of resorcinol in an ice-water bath, stir at room temperature for 1h, and then react at 60°C for 3 hours. After the reaction was complete, water (300 mL) was added, stirred at room temperature for 1 h, and filtered under reduced pressure. The obtained white solid was washed three times with water (100 mL), and dried to obtain the product (17.6 g, yield 67%).
[0288] (2) Preparation of 2-(6-hydro...
Embodiment 2
[0317] Example 22-(6-((4'-(2-(cyclopropylsulfonylamino)ethoxy)-2',6'-dimethyl-[1,1'-biphenyl]-3 Preparation of -yl)methoxy)-2,3-dihydrobenzofuran-3-yl)acetic acid (compound 2)
[0318]
[0319] (1) Preparation of 2-(4-bromo-3,5-dimethylphenoxy)ethylamine
[0320]
[0321] Dissolve tert-butyl 2-(4-bromo-3,5-dimethylphenoxy)ethylcarbamate (2.0 g, 5.8 mmol) in dichloromethane (20 mL) and add trifluoroacetic acid (10 mL) , stirred at room temperature for 2h. The solvent was distilled off under reduced pressure, saturated sodium bicarbonate solution was added, extracted with ethyl acetate (100mL×3), the organic phases were combined, washed with saturated aqueous sodium chloride solution, dried over anhydrous sodium sulfate, and the solvent was distilled off under reduced pressure to obtain an oily product (1.36 g, yield 96%).
[0322] (2) Preparation of N-(2-(4-bromo-3,5-dimethylphenoxy)ethyl)cyclopropylsulfonamide
[0323]
[0324] Dissolve 2-(4-bromo-3,5-dimethylphen...
Embodiment 3
[0336]Example 32-(6-((4'-(2-((N,N-dimethylaminosulfonyl)amino)ethoxy)-2',6'-dimethyl-[1,1'- Preparation of biphenyl]-3-yl)methoxy)-2,3-dihydrobenzofuran-3-yl)acetic acid (compound 3)
[0337]
[0338] (1) Preparation of tert-butyl 2-(4-bromo-3,5-dimethylphenoxy)ethylcarbamate
[0339]
[0340] N-Boc ethanolamine (5.8g, 36mmol), 4-bromo-3,5-dimethylphenol (6.03g, 30mmol), and azodicarbonyldipiperidine (11.34g, 45mmol) were dissolved in tetrahydrofuran ( 300mL), add tri-n-butylphosphine (9.1g, 45mmol) under ice bath, rise to room temperature after dropwise addition, react for 16h, add petroleum ether (200mL), suction filter, filtrate is spin-dried, crude product is passed through silica gel column Chromatography (ethyl acetate / petroleum ether=0~1 / 20) separated the product as a colorless oil (8.0 g, yield 77%).
[0341] (2) Preparation of 2-(4-bromo-3,5-dimethylphenoxy)ethylamine
[0342]
[0343] Dissolve tert-butyl 2-(4-bromo-3,5-dimethylphenoxy)ethylcarbamate (2.5 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com