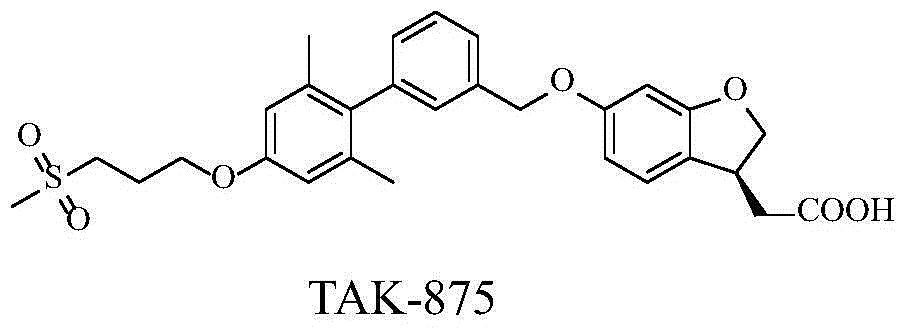
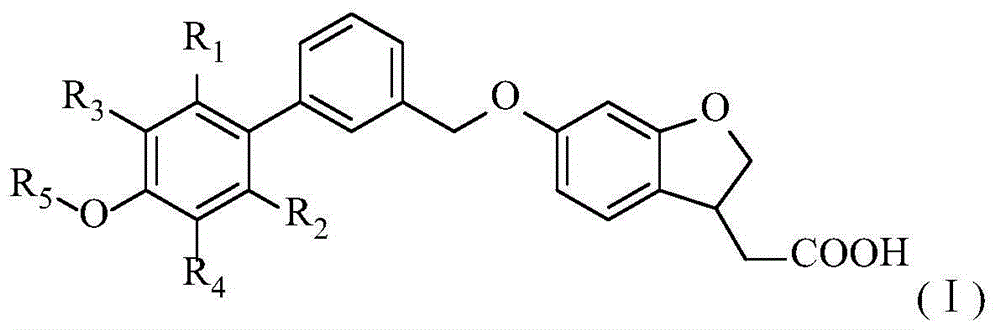
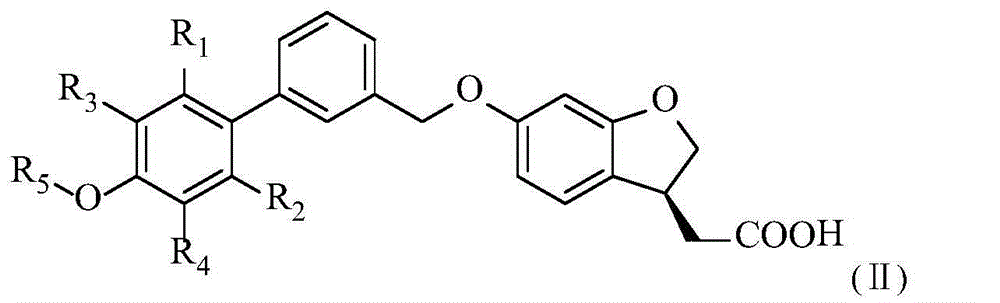
Aromatic polycyclic carboxylic acid derivatives
A technology of compounds and substituents, applied in the field of aromatic polycyclic carboxylic acid derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0255] Example 12-(6-((2'-cyclopropyl-4'-((tetrahydrofuran-3-yl)oxy)-[1,1'-biphenyl]-3-yl)methoxy)- Preparation of 2,3-dihydrobenzofuran-3-yl)acetic acid (compound 1)
[0256]
[0257] (1) Preparation of methyl 2-(6-((3-bromobenzyl)oxy)-2,3-dihydrobenzofuran-3-yl)acetate
[0258]
[0259] 2-(6-Hydroxy-2,3-dihydrobenzofuran-3-yl)methyl acetate (1.04g, 5.0mmol) and 3-bromobenzyl alcohol (935mg, 5.0mmol) and azodicarbonyl Dipiperidine (1.89 g, 7.5 mmol) was dissolved in anhydrous THF (30 mL), and stirred thoroughly for 30 minutes. Dissolve n-butylphosphine (1.51g, 7.5mmol) in anhydrous tetrahydrofuran (30mL), add it dropwise to the reaction system with a dropping funnel, stir fully for 2 hours, and the reaction solution is directly concentrated under reduced pressure and then chromatographed on a silica gel column ( The eluent petroleum ether / ethyl acetate=15:1) isolated the product 1.1g, and the yield was 58%.
[0260] (2) 2-(6-((3-(4,4,5,5-tetramethyl-1,3,2-dioxaborola...
Embodiment 2
[0283] Example 22-(6-((2'-cyclopropyl-4'-(2-(methylsulfonylamino)ethoxy)-[1,1'-biphenyl]-3-yl)methoxy base)-2,3-dihydrobenzofuran-3-yl)acetic acid (compound 3)
[0284]
[0285] (1) Preparation of 2-(tert-butoxycarbonylamino) ethyl methanesulfonate
[0286]
[0287] Dissolve N-tert-butoxycarbonylethanolamine (6.2g, 38.5mmol) in anhydrous tetrahydrofuran (150mL), add triethylamine (9.7g, 96mmol), add methanesulfonyl chloride (5.3g, 46mmol) dropwise under ice-cooling, The dropwise addition was completed within 20 minutes, and the reaction was continued for 12 hours at room temperature. The system was filtered under reduced pressure, and the filtrate was concentrated under reduced pressure, then extracted with ethyl acetate and water, the organic phase was dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain 7.7 g of the title compound as a brown oil, with a yield of 83.7%.
[0288] (2) Preparation of tert-butyl 2-(4-bromo-3-formylphenoxy...
Embodiment 3
[0314] Example 3 (S)-2-(6-((2'-cyclopropyl-4'-(2-(1,1-dioxoisothiazolidin-2-yl)ethoxy)-[1 ,1'-biphenyl]-3-yl)methoxy)-2,3-dihydrobenzofuran-3-yl)acetic acid (compound 6)
[0315]
[0316] (1) Preparation of 2-(tert-butoxycarbonylamino) ethyl methanesulfonate
[0317]
[0318] Dissolve N-tert-butoxycarbonylethanolamine (15g, 93mmol) in anhydrous tetrahydrofuran (400mL) under ice-cooling, add triethylamine (23.5g, 233mmol), add dropwise methanesulfonyl chloride (12.8g, 111.6mmol), drop After the addition was complete, it was raised to room temperature and reacted for 12 hours. Filtrate with suction, concentrate the filtrate under reduced pressure, add ethyl acetate (300mL) and water (300mL), extract with ethyl acetate (300mL×3), dry the organic phase with anhydrous sodium sulfate, and concentrate under reduced pressure to give the title compound as a brown oil 18.9 g, 85% yield.
[0319](2) Preparation of tert-butyl 2-(4-bromo-3-formylphenoxy)ethylcarbamate
[0320] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com