Aminopyrrole zinc-lithium bimetallic catalyst, preparation method and application thereof
A bimetallic catalyst, zinc aminopyrrole technology, applied in the direction of organic chemistry, etc., can solve the problems of difficult controllable polymerization, low catalytic efficiency, limited application, etc., and achieve the effects of high catalytic activity, simple preparation process, and uniform molecular weight distribution.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
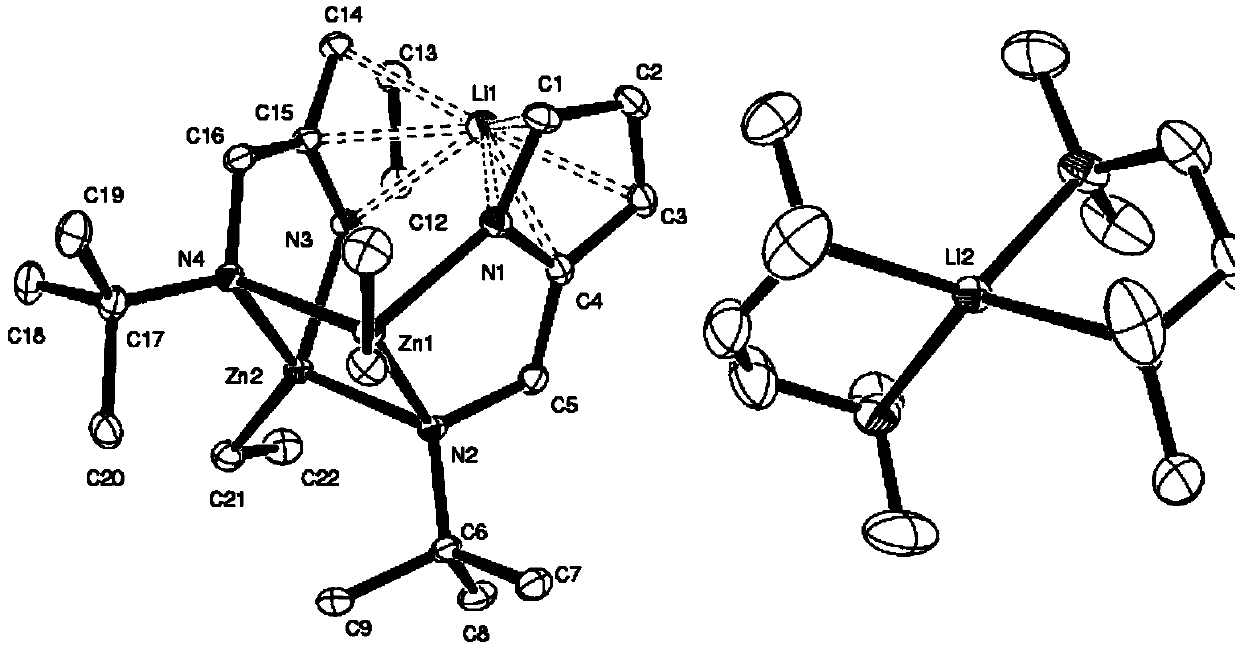
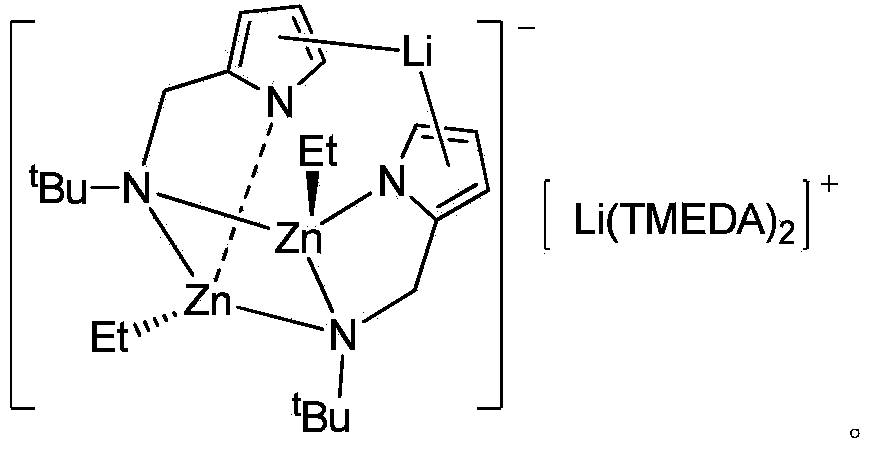
[0017] Preparation and Characterization of Embodiment 1 Aminopyrrole Zinc Lithium Bimetallic Catalyst
[0018] (1) Preparation of aminopyrrole ligand
[0019] In a three-neck round bottom flask, add 0.1 mol formaldehyde aqueous solution, cool to 0°C, then add 0.1 mol tert-butylamine hydrochloride, and control the temperature at 0-5°C. After stirring for 10 minutes, continue to cool to 0°C, add 0.1mol pyrrole dropwise, control the temperature at 0°C and stir for 1 hour, slowly return to room temperature, and continue stirring overnight. After the reaction was completed, 20% sodium hydroxide was added for neutralization, the aqueous phase was extracted with ether, dried over anhydrous magnesium sulfate, the solvent was spin-dried, and recrystallized to obtain 2-tert-butylaminomethylpyrrole ligand with a yield of 70%.
[0020] (2) Preparation of aminopyrrole zinc-lithium bimetallic catalyst
[0021] Under the protection of an inert gas and an ice-water bath, a n-butyllithium so...
Embodiment 2
[0025] (1) the preparation of catalyst is the same as embodiment 1
[0026] (2) Lactide ring-opening polymerization: Dissolve lactide (7.20g, 50mmol) and catalyst (0.1mmol) in tetrahydrofuran solvent, and after the solution is clarified, add a few drops of 5% n-butanol in tetrahydrofuran solution to carry out Initiate, react at room temperature for 5 hours, use anhydrous acetic acid to terminate the reaction, then slowly add absolute ethanol to precipitate polylactic acid, and filter to obtain a colorless and transparent polylactic acid product. The conversion rate is 95%, the PDI=1.19, and the glass transition temperature is 107.
Embodiment 3
[0028] (1) the preparation of catalyst is the same as embodiment 1
[0029](2) Lactide ring-opening polymerization: Dissolve lactide (8.65g, 60mmol) and catalyst (0.1mmol) in tetrahydrofuran solvent, and after the solution is clarified, add a few drops of 5% n-butanol in tetrahydrofuran solution to carry out Initiate, after reacting at room temperature for 5 hours, use anhydrous acetic acid to terminate the reaction, then slowly add absolute ethanol to precipitate polylactic acid, filter to obtain a colorless and transparent polylactic acid product. The conversion rate is 91%, PDI=1.23, and the glass transition temperature is 98.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com