Preparation method of core-shell structured catalyst for direct hydroboron fuel cell anode
A borohydride, fuel cell technology, used in fuel cells, battery electrodes, nanotechnology for materials and surface science, etc. Catalytic oxidation activity, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
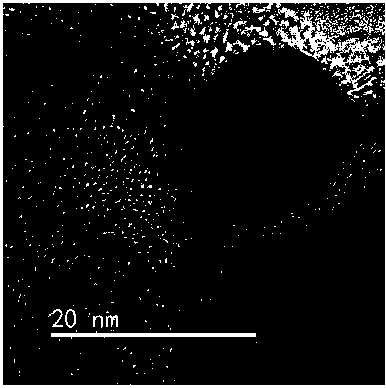
[0041] Preparation of Cu-Ni alloy nanoparticles: the preparation concentration is 0.5 mol L -1 NiCl 2 and CuCl 2 Aqueous solution, respectively take 0.5mL and successively add 30 drops·min -1 The dropping speed was added to the mixed solution containing 4mL polyoxyethylene lauryl ether and 32 mL n-octane, at this time, ω=9, x=8, argon gas was always passed through during the dropping process and mechanical stirring and ultrasonic dispersion were maintained. Formulated as an inverse microemulsion containing two metal precursors. In the same way as above, 1ml of 2.0 mol L ?1 NaBH 4 In the mixed solution of aqueous solution, 4ml polyoxyethylene lauryl ether and 32ml n-octane, configure the reducing agent inverse microemulsion, and then reduce the reducing agent inverse microemulsion with 30 drops min -1 Add the reactant microemulsion at a dropping speed of 100%. During the dropping process, argon gas is always passed through and mechanical stirring and ultrasonic dispersion ...
Embodiment 2
[0046] Preparation of Cu-Fe alloy nanoparticles: the preparation concentration is 0.5 mol L -1 CuCl 2 and FeCl 2 Aqueous solution, respectively take 0.5mL and successively add 30 drops·min -1 The dropping speed was added to the mixed solution containing 3 mL polyoxyethylene stearyl ether and 18 mL n-heptane, at this time ω=7, x=6, argon gas was always passed through during the dropping process and mechanical stirring was maintained. Ultrasonic dispersion, formulated as an inverse microemulsion containing two metal precursors. In the same way as above, 1ml of 2.0 mol L ?1 hydrazine aqueous solution, 3 ml of polyoxyethylene stearyl ether and 18 ml of n-heptane mixed solution, configured as a reducing agent inverse microemulsion, and then the reducing agent inverse microemulsion was mixed with 30 drops·min -1 Add the reactant microemulsion at a dropping speed of 100%. During the dropping process, argon gas is always passed through and mechanical stirring and ultrasonic disper...
Embodiment 3
[0051] Preparation of Cu-Zn alloy nanoparticles: the preparation concentration is 0.5 mol L -1 CuCl 2 and ZnCl 2 Aqueous solution, respectively take 0.5mL and successively add 30 drops·min -1 The dropping speed is added to the mixed solution containing 6 mL sodium sulfosuccinate and 30 mL isooctane, at this time, ω=4, x=5, and argon gas is always passed through during the dropping process, and mechanical stirring and ultrasonic dispersion are maintained. , formulated as an inverse microemulsion containing two metal precursors. In the same way as above, 1ml of 2.0 mol L ?1 NaBH 4 Aqueous solution mixed with 6 ml sodium sulfosuccinate and 30 ml isooctane to form a reducing agent inverse microemulsion, and then reduce the reducing agent inverse microemulsion with 30 drops min -1 Add the reactant microemulsion at a dropping speed of 100%. During the dropping process, argon gas is always passed through and mechanical stirring and ultrasonic dispersion are maintained for 30 min...
PUM
| Property | Measurement | Unit |
|---|---|---|
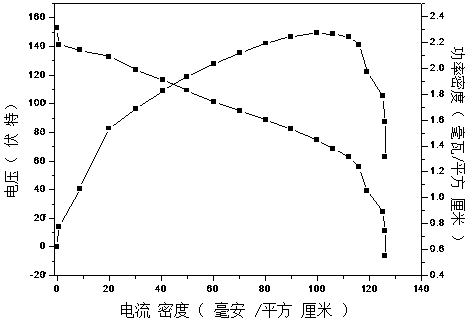
| Maximum power density | aaaaa | aaaaa |
| Maximum power density | aaaaa | aaaaa |
| Maximum power density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com