Protease
A technology of protease and enzymatic activity, applied in the field of Deinococcus radiodurans protease, which can solve undiscovered problems and achieve the effect of high specificity and good heat resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0037] (1) Protease activity and substrate cleavage sequence specificity of PprI
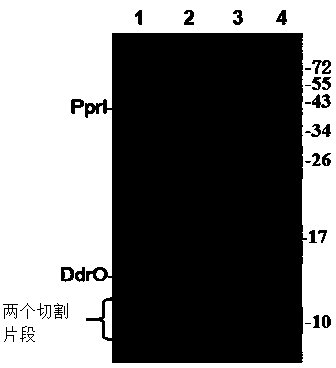
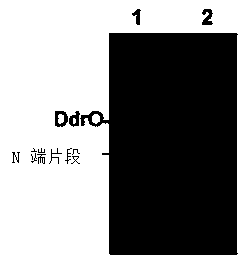
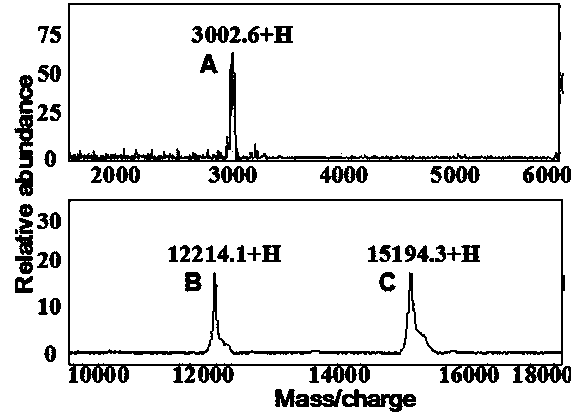
[0038] The purified PprI protein and its substrate protein DdrO were mixed in reaction buffer (100mM NaCl, 30mM Tris-HCl 8.0, 1mM DTT, 3.0mM MnCl 2 ) for 40 minutes. Through SDS-PAGE electrophoresis and mass spectrometry molecular weight detection, the PprI protein can cut the DdrO enzyme into two segments. The specific sequences of PprI protease cleavage substrates were ELRGKR, ELRGAR, ELAGKR and ELAGAR by point mutation of the amino acid residues near the DdrO cleavage site. In addition, the cleavage position can be obtained between the second and third amino acid residues by mass spectrometry sequencing of the C-terminal of the protein.
[0039](2) The optimum temperature range and temperature resistance of PprI protease activity
[0040] The optimum temperature for the enzymatic cleavage reaction between PprI protease and substrate is between 35-40°C. Protease activity is highest in...
Embodiment 3
[0042] (1) Protease activity and substrate cleavage sequence specificity of PprI
[0043] The purified PprI protein and its substrate protein DdrO were mixed in reaction buffer (200mM NaCl, 20mM Tris-HCl 8.0, 1mM DTT, 5.0mM MnCl 2 ) for 40 minutes. Through SDS-PAGE electrophoresis and mass spectrometry molecular weight detection, the PprI protein can cut the DdrO enzyme into two segments. Through the point mutation of the amino acid residues near the DdrO restriction site, the specific sequences of the PprI protease digestion substrates are ELRGKR, ELRGAR, ELRGER, ELAGKR, ELAGAR and ELAGER. In addition, the cleavage position can be obtained between the second and third amino acid residues by mass spectrometry sequencing of the C-terminal of the protein.
[0044] (2) The optimum temperature range and temperature resistance of the protease activity of PprI
[0045] The optimum temperature for the enzymatic cleavage reaction between PprI protease and substrate is between 35-4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com