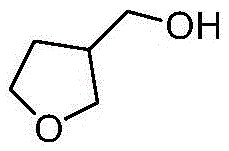
A kind of synthetic method of 3-hydroxymethyl tetrahydrofuran
A technology of hydroxymethyltetrahydrofuran and synthesis method, applied in the direction of organic chemistry, etc., can solve the problems of low total yield, high cost of reaction raw materials, large amount of metal borohydride used, etc., to reduce the production of by-products and the cost of preparation Reduction, the effect of usage reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
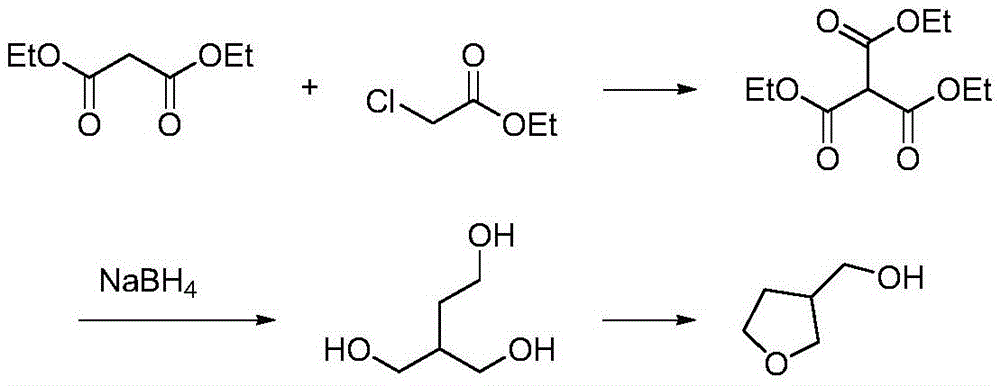
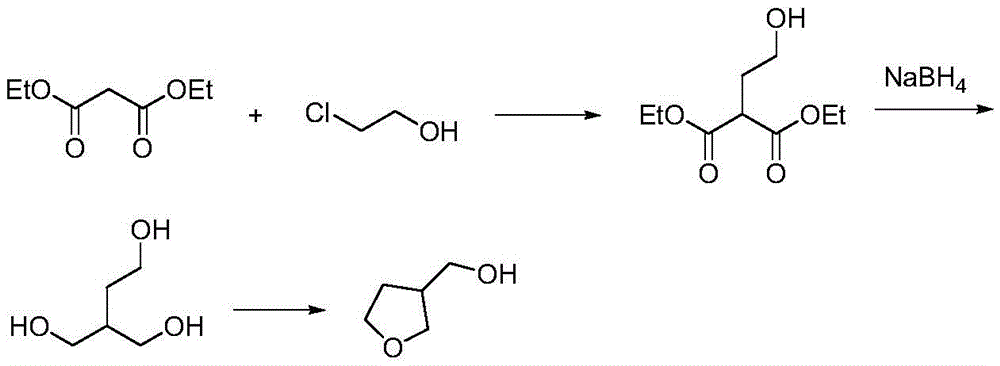
[0018] (1) In a 500ml reaction flask, after adding 80g of diethyl malonate, add dropwise 200ml of ethanol solution of 56g of potassium tert-butoxide, and slowly heat to 80°C to react. After dropping, drop slowly Add 40.3 grams of 2-chloroethanol, drop it, and react at a temperature of 80°C for 7 hours, evaporate the ethanol solvent, and filter to obtain 96 grams of intermediate 2-hydroxyethyl-diethyl succinate, with a yield of 95%. Purity 96% (HPLC).
[0019] IR :3638,3462,2984,1736,1174,859cm -1 .
[0020] (2) 96 grams of 2-hydroxyethyl-diethyl succinate prepared in step (1) are dropped into a 500 milliliter reaction flask, add 200 milliliters of methanol as a solvent, then add 36 grams of boron in batches Sodium hydride, temperature controlled at 70°C, reacted for 2 hours, and reduced to obtain 43 g of crude 2-hydroxymethyl-1,4-butanediol with a yield of 80% and a purity of 98% (HPLC).
[0021] 1 HNMR (D 2 O,δ:ppm):δ1.53(2H,q,J=6Hz,CH CH 2 ),1.75(1H,m, CHCH 2 ),3...
Embodiment 2
[0028] (1) In a 1000 ml reaction flask, after adding 261 grams of diethyl malonate, 200 ml of ethanol solution of 62 grams of sodium methoxide was added dropwise, and slowly heated to 75°C to react. After dropping, slowly add 132 grams of 2-Chloroethanol, after dripping, reacted at 75°C for 6 hours, evaporated the ethanol solvent, cooled and filtered, and removed sodium chloride to obtain 312.5 grams of 2-hydroxyethyl-diethyl succinate with a yield of 95 %, purity 96% (HPLC).
[0029] (2) 96 grams of intermediate 2-hydroxyethyl-diethyl succinate obtained in step (1) are dropped into a 500-ml reaction flask, and 200 ml of methyl alcohol is added as a solvent, and then 36 1 g of sodium borohydride was reacted at a temperature of 70° C. for 2 hours, and then reduced to obtain 41.9 g of crude 2-hydroxymethyl-1,4-butanediol with a yield of 80% and a purity of 98% (HPLC).
[0030] (3) get the 2-hydroxymethyl-1,4-butanediol that makes in step (2) and can add in the 500 milliliters t...
Embodiment 3
[0032] (1) In a 1000 ml reaction flask, after adding 160 grams of diethyl malonate, dropwise add 52 grams of ethanol solution of sodium ethylate, and slowly heat to 60°C for reaction, after dropping, slowly add 89 grams of 2- Chlorohydrin, dropwise, reacted at 60°C for 10 hours at a temperature of 60°C, evaporated the ethanol solvent, and filtered to obtain 194 g of 2-hydroxyethyl-diethyl succinate with a yield of 96% and a purity of 97% (HPLC).
[0033] (2) Get 96 grams of 2-hydroxyethyl-diethyl succinate prepared in step (1) and drop into a 500-ml reaction flask, add 200 ml of methanol as a solvent, then add 52 grams of boron in batches Potassium hydride was reacted at a temperature of 60°C for 2 hours, and 41 g of crude 2-hydroxymethyl-1,4-butanediol was obtained by reduction, with a yield of 80% and a purity of 97% (HPLC).
[0034] (3) 2-hydroxymethyl-1,4-butanediol prepared in step (2) can be added in the 500 milliliter three-neck reaction flask that water trap and reflux...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com