Naringin and fexofenadine hydrochloride drug composition and preparation thereof
A composition and technology of naringin, applied in the field of naringin pharmaceutical composition and preparation thereof, can solve problems such as adverse reactions, death and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
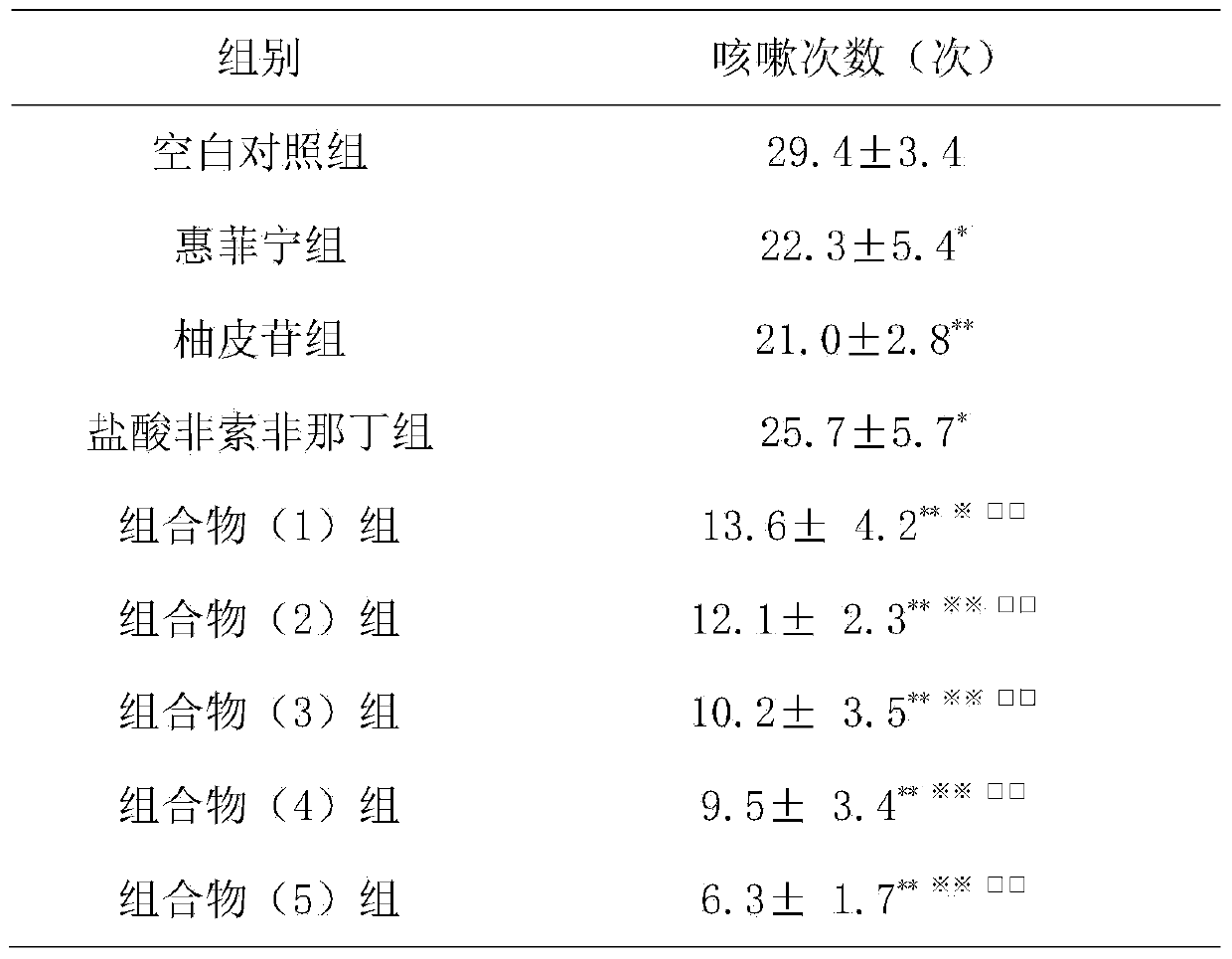
[0012] Example 1: Inhibition of cough in guinea pigs caused by citric acid
[0013] 1. Materials
[0014] 1.1 Experimental animals Qualified Hartley guinea pigs, weighing 250-300g, male and female, were provided by the Guangdong Medical Experimental Animal Center.
[0015] 1.2 Drugs and reagents: Huifeining; naringin is prepared according to the daily dosage of 120 mg; fexofenadine hydrochloride is prepared according to the daily dosage of 6 mg; the composition (1) group is prepared according to the daily dosage of 27.5 mg of naringin and fexofenadine hydrochloride. 30 mg of phenadine was prepared; the composition (2) group was prepared according to the daily dosage of naringin 27.5 mg and fexofenadine hydrochloride 300 mg; the composition (3) group was prepared according to the daily dosage of naringin 275 mg and fexofex hydrochloride Natin 30mg preparation; composition (4) group is prepared according to human daily dosage of naringin 275mg, fexofenadine hydrochloride 300mg;...
Embodiment 2
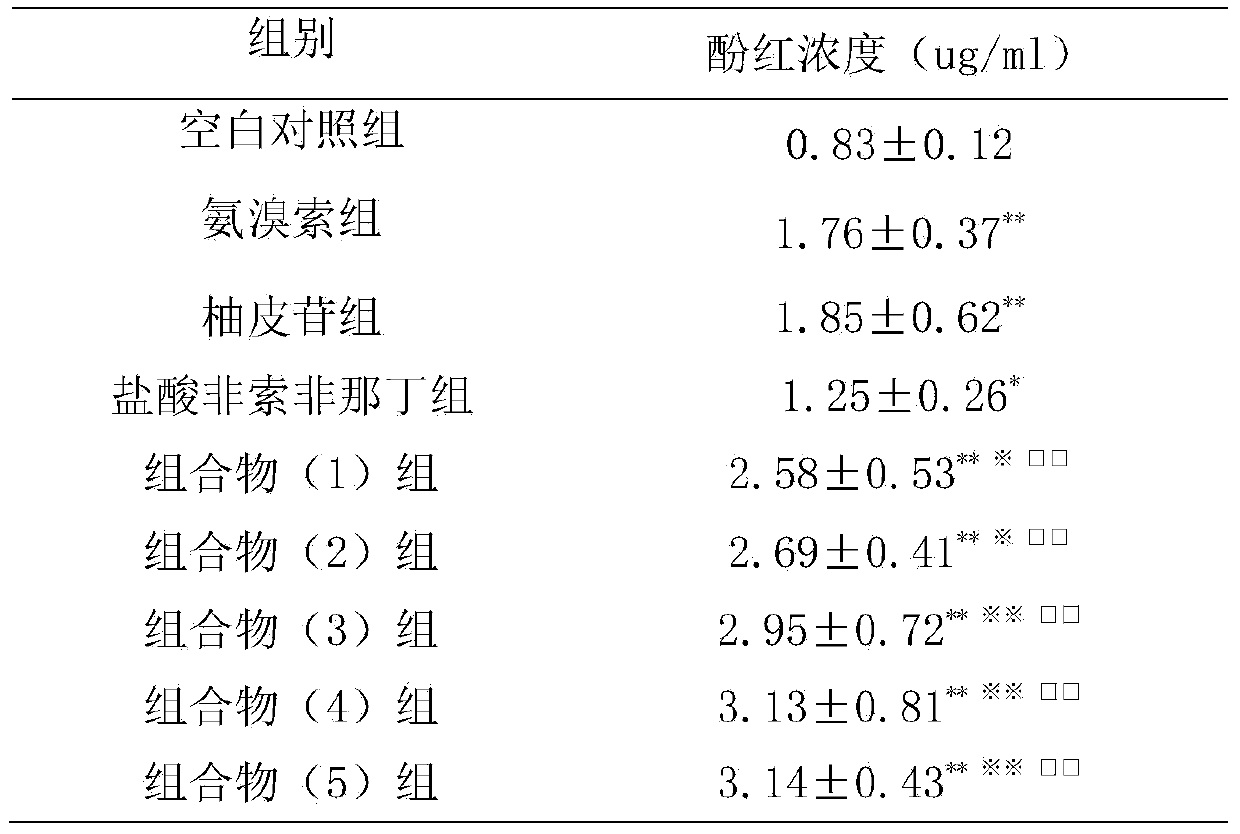
[0027] Example 2: Effects on Mouse Phenol Red Excretion Experiment
[0028] 1. Materials
[0029] 1.1 Experimental animals Kunming mice, half male and half male, weighing 30-40 g, were provided by the Guangdong Medical Experimental Animal Center.
[0030] 1.2 Drugs and reagents Ambroxol; naringin is prepared according to the daily dosage of 120 mg; fexofenadine hydrochloride is prepared according to the daily dosage of 120 mg; the composition (1) group is prepared according to the daily dosage of naringin 27.5 mg, fexofox 30 mg of phenadine was prepared; the composition (2) group was prepared according to the daily dosage of naringin 27.5 mg and fexofenadine hydrochloride 300 mg; the composition (3) group was prepared according to the daily dosage of naringin 275 mg and fexofex hydrochloride Natin 30mg preparation; composition (4) group is prepared according to human daily dosage of naringin 275mg, fexofenadine hydrochloride 300mg; composition (5) group is prepared according ...
Embodiment 3
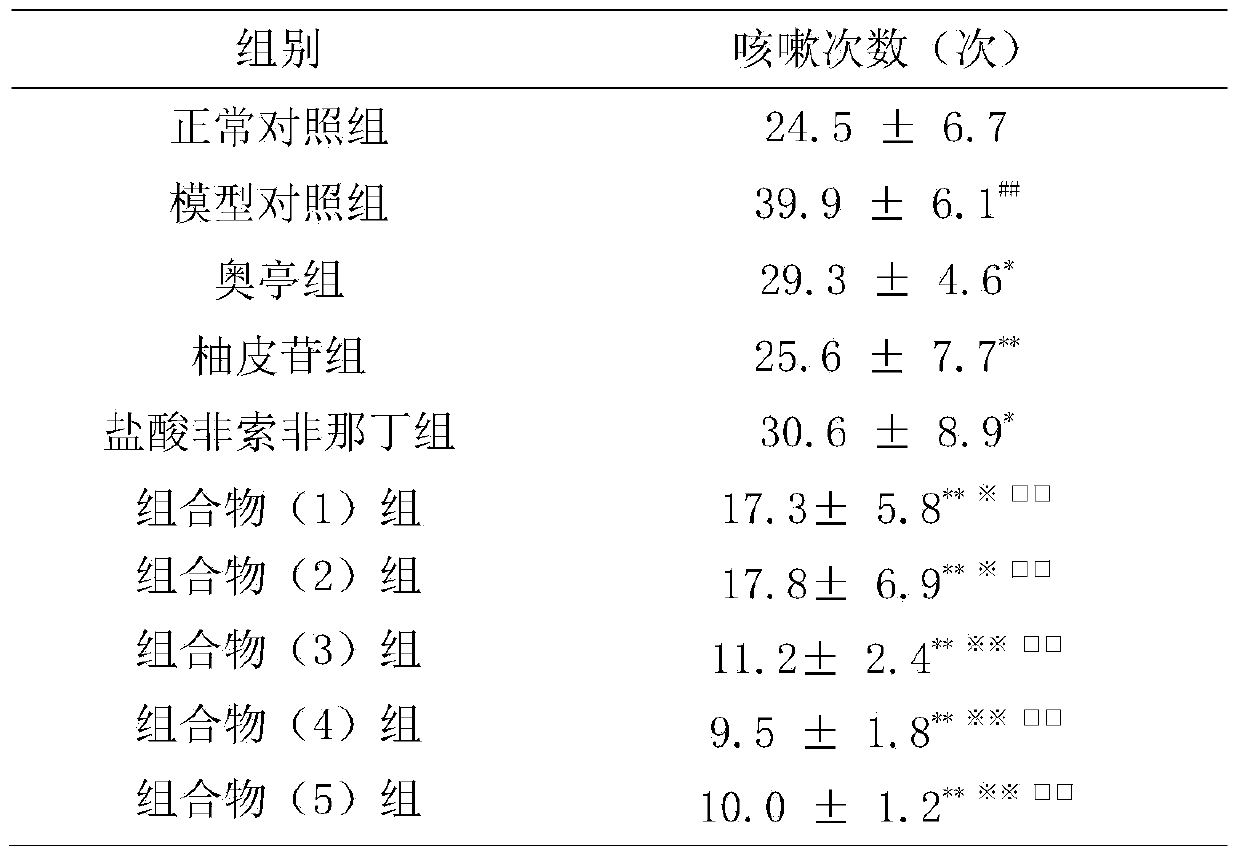
[0043] Example 3: Effect on Allergic Cough (Cough Variation Asthma) Induced by Ovalbumin
[0044] 1. Materials
[0045] 1.1 Experimental animals: Hartley guinea pig, male, weighing 250-300 g, SPF grade, provided by Guangdong Medical Experimental Animal Center.
[0046]1.2 Drugs and reagents Cyclophosphamide; Ovalbumin; Capsaicin; Methacholine; Aoting cough syrup (compound codeine phosphate solution); The daily dose of human is 120 mg; the composition (1) group is prepared according to the daily dose of naringin 27.5 mg and fexofenadine hydrochloride 30 mg; the composition (2) is prepared according to the daily dose of naringin 27.5 mg and fexofenadine hydrochloride 300 mg of phenadine was prepared; the composition (3) group was prepared according to the daily dose of naringin 275 mg and fexofenadine hydrochloride 30 mg; the composition (4) group was prepared according to the daily dose of naringin 275 mg and fexofenadine hydrochloride Ding 300mg preparation; composition (5) ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com