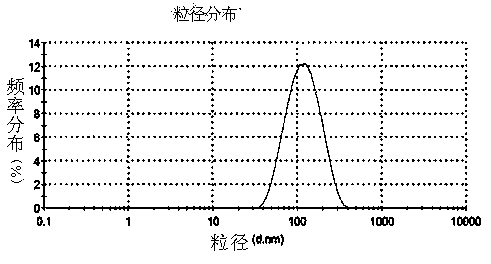
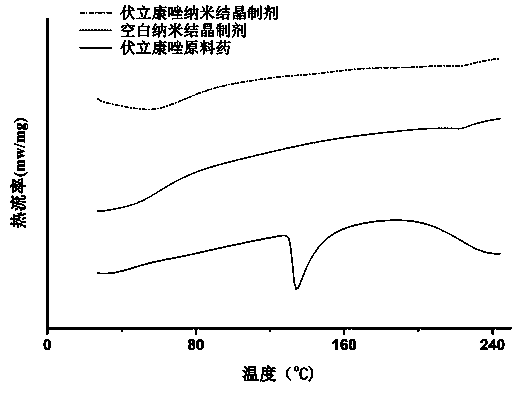
Eye use voriconazole nanocrystal preparation and preparation method thereof
A voriconazole ophthalmic and nanocrystalline technology, which is applied in the field of voriconazole ophthalmic nanocrystalline preparations and its preparation, can solve the problems of limited effect and poor solubility of voriconazole, and achieves the effects of good stability, reduced adverse reactions, and easy industrialized production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] (1) Disperse 5 g of voriconazole and 50 g of fat-soluble stabilizer in 50 ml of organic solvent to obtain drug solution A; wherein, the fat-soluble stabilizer is Eudragit RS100 or EVA, and the organic solvent is an acetone-methanol mixture with a volume ratio of 3:1 ;
[0042] (2) Disperse 10g of water-soluble stabilizer and 20g of penetration enhancer in 600ml of distilled water to obtain an aqueous dispersion medium, and obtain aqueous dispersion medium solution B, wherein the water-soluble stabilizer is PVA or PVP, and the penetration enhancer is N -Methylpyrrolidone (Pharmasolve) and sodium hyaluronate, the ratio of the two penetration enhancers is 9:1;
[0043] (3) Disperse the drug solution A prepared in step (1) in the aqueous dispersion medium B prepared in step (2) under high-speed shear or ultrasonic conditions to obtain suspension C; wherein the high-speed shear condition It is a Fluko-FA25 high-shear dispersing and emulsifying machine, shearing at 13000rpm ...
Embodiment 2
[0048] (1) Disperse 0.5 g of voriconazole and 10 g of glyceryl monooleate in 20 ml of an organic solvent to obtain drug solution A; wherein the organic solvent is an acetone-PEG400 mixture with a volume ratio of 1:1;
[0049] (2) Disperse 40g of water-soluble stabilizer and 20g of penetration enhancer in 800ml of distilled water to obtain aqueous dispersion medium B, wherein the water-soluble stabilizer is Poloxamer 407, and the penetration enhancer is sodium hyaluronate and propylene glycol , the ratio of the two penetration enhancers is 1:4;
[0050] (3) Disperse the drug solution A prepared in step (1) in the aqueous dispersion medium B prepared in step (2) under high-speed shear or ultrasonic conditions to obtain suspension C; wherein the high-speed shear condition It is a Fluko-FA25 high-shear dispersing and emulsifying machine, shearing at 8000rpm for 10 minutes, the ultrasonic conditions are power 400-900W, ultrasonic ratio 2:2, ultrasonic frequency 30 times;
[0051] ...
Embodiment 3
[0055] (1) Disperse 20.0g of voriconazole and 100g of fat-soluble stabilizer in 200ml of organic solvent to obtain drug solution A; wherein the fat-soluble stabilizer is Eudragit RL100 and ethylene-vinyl acetate polymer (EVA), two fat-soluble stabilizers The solvent ratio is 4:1; the organic solvent is an acetone-ethanol mixture with a volume ratio of 2:1;
[0056] (2) Disperse 50g of water-soluble stabilizer and 20g of penetration enhancer in 600ml of distilled water to obtain aqueous dispersion medium B, wherein the water-soluble stabilizer is PVA, and the penetration enhancer is sodium lauryl sulfate and propylene glycol, two The ratio is 3:1; (3) Disperse the drug solution A prepared in step (1) in the aqueous dispersion medium B prepared in step (2) under high-speed shear or ultrasonic conditions to obtain suspension C Wherein the condition of high-speed shearing is Fluko-FA25 high-shear dispersing emulsifier, 13000rpm shearing 15min, the condition of ultrasonic is power ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com