Anti-tumor phosphatase and tensin homolog deleted on chromosome ten (PTEN)-VP22 gene
A PTEN-VP22, anti-tumor technology, applied in the field of genetic engineering, can solve the problems of low PTEN gene transduction efficiency, hindering the clinical application of PTEN gene therapy, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1—construct anti-tumor PTEN-VP22 gene by way of PCR
Embodiment 2
[0072] The PTEN-VP22 gene of the control example 1-3 obtained in embodiment 2-embodiment 1 is for the cell in vitro experiment of tumor cell
[0073] The PTEN-VP22 gene of Control Example 1-3, as well as PTEN and VP22 genes were connected into eukaryotic expression plasmid pcDNA3 to construct pcDNA3-PTEN-VP22, pcDNA3-PTEN and pcDNA3-VP22 expression vectors respectively.
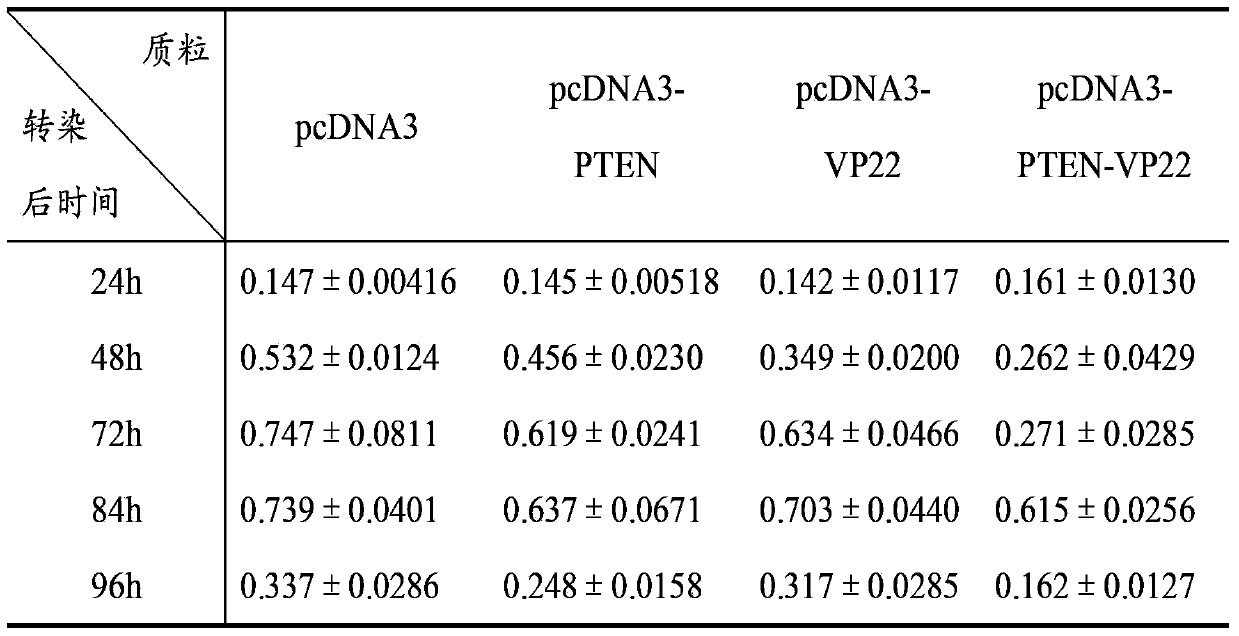
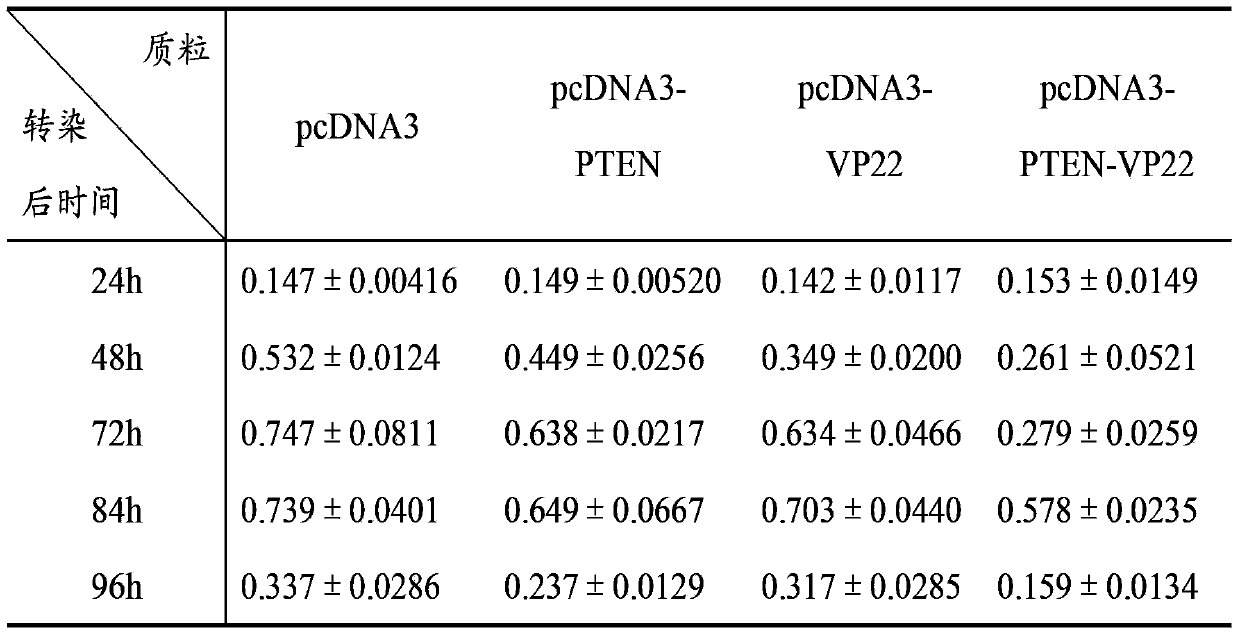
[0074] Cell experiment 1: Inhibitory effect on breast cancer cell line BT549
[0075] A. Time-effect relationship: Transfect 1 μg of pcDNA3, pcDNA3-PTEN, pcDNA3-VP22, and pcDNA3-PTEN-VP22 plasmids into BT549 cells cultured in a 24-well plate, and set up a negative control group of untransfected BT549 cells. 8 replicate wells. Cells were cultured for 4 h after transfection, and 100 μl of cell suspension (1×10 3 cells), at 37°C, 5% CO 2 Cultivate under conditions; after 24h, 48h, 72h, 84h, and 96h, add 10 μl of CCK-8 solution to each well at 37°C, 5% CO 2 After incubation for 2 hours, the A450 value was mea...
Embodiment 3
[0118] The PTEN-VP22 gene of control example 1 obtained in embodiment 3-embodiment 1 is to the animal experiment of tumor cell
[0119] The PTEN-VP22 gene and PTEN of Control Example 1 were connected into the eukaryotic expression plasmid pcDNA3 to construct pcDNA3-PTEN-VP22 and pcDNA3-PTEN expression vectors respectively.
[0120] Animal Experiment 1: Inhibitory Effect on Breast Cancer Cells
[0121] Breast cancer cells BT549 were inoculated subcutaneously in nude mice to establish tumor-bearing nude mice, and the tumor-bearing nude mice were randomly divided into three groups: experimental group 1, experimental group 2 and negative control group. Experimental group 1 was injected intratumorally with 500uL, 100μg / mL pcDNA3-PTEN-VP22 gene expression vector, experimental group 2 was injected with 500uL, 100μg / mL pcDNA3-PTEN gene expression vector, and the negative control group was not injected. Observe the survival rate and average tumor volume of the three groups of mice on ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com