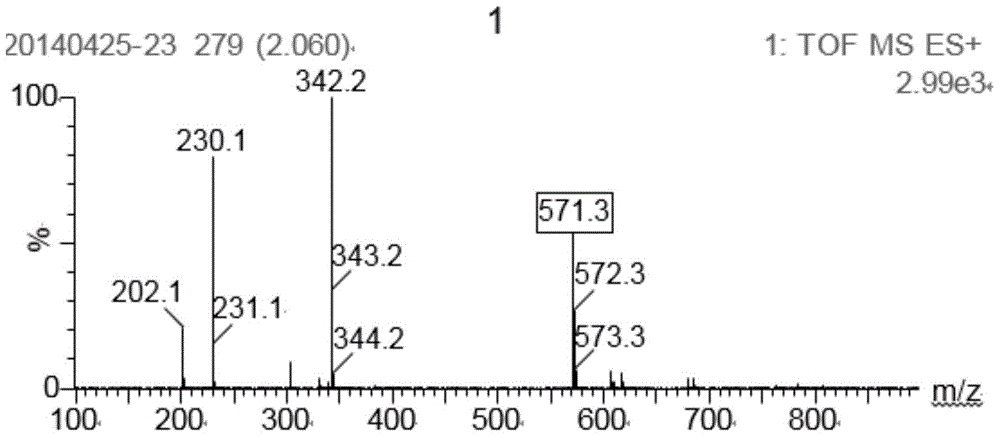
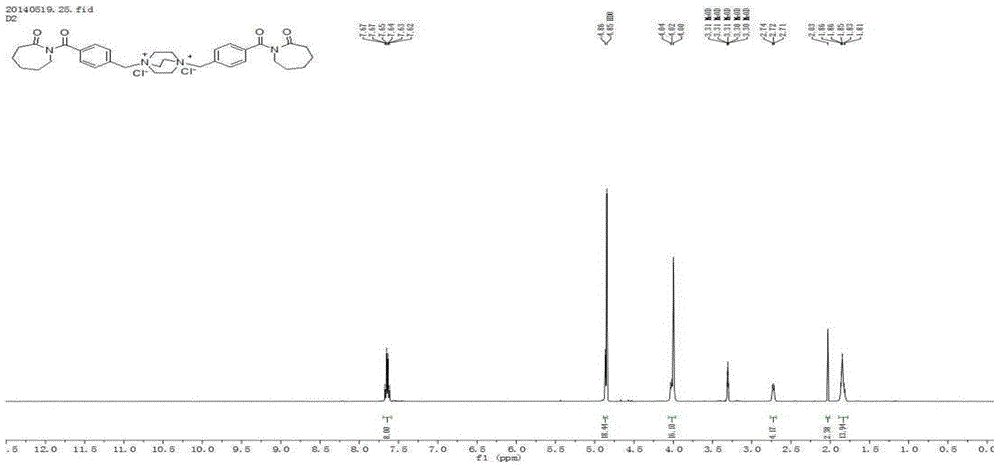
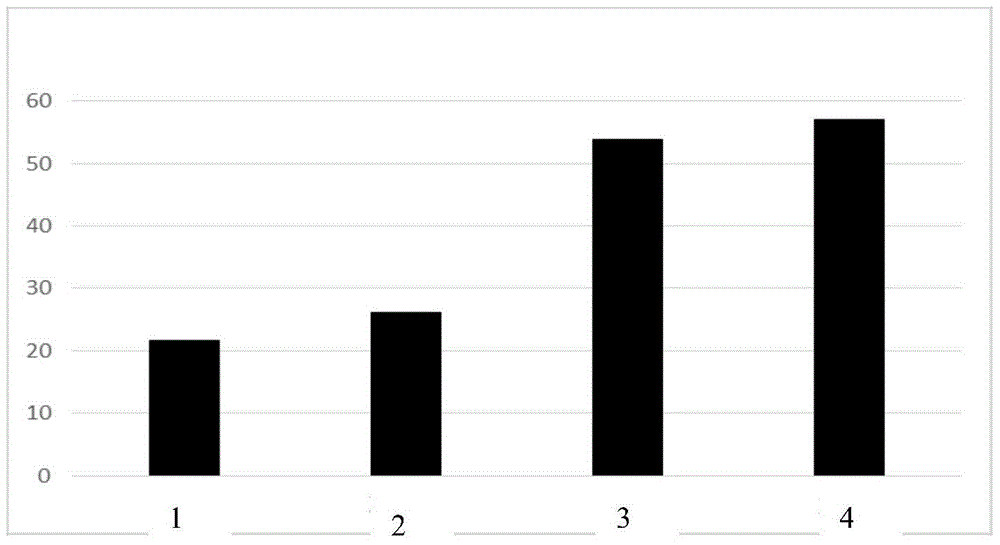
A kind of double cationic bleach activator and its synthesis method and application
A bleaching activator and dicationic technology, which is applied in the field of dicationic bleaching activator and its synthesis, can solve the problem that there is no dicationic bleaching activator, etc., and achieve the effect of good bleaching effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] 1. Synthesis of 4-chloromethylbenzoyl valerolactam
[0033] Add 0.1 mol of valerolactam into a 500 mL three-neck flask filled with 0.15 mol of triethylamine and 110 mL of toluene, and after being protected by argon, slowly heat to boiling and reflux under magnetic stirring. Slowly add 50 mL of toluene solution dissolved with 0.1 mole of 4-chloromethylbenzoyl chloride into a three-necked flask, condense and reflux for 6 hours under magnetic stirring. After the reaction is complete, cool to room temperature. After filtration, the filtrate was refrigerated overnight. The precipitated white precipitate was filtered off, washed with cold toluene and dried in vacuum for use.
[0034] 2. N-[4-(triethylammoniummethylene)benzoyl]caprolactam chloride
[0035] Dissolve 0.1 mole of 4-chloromethylbenzoyl valerolactam in 150mL of acetonitrile, under the protection of argon, slowly heat to boiling under magnetic stirring and reflux, then dissolve 50mL of acetonitrile solution with ...
Embodiment 2
[0037] This embodiment provides a kind of synthetic method of double cationic bleach activator, and concrete steps are as follows:
[0038] 1. Synthesis of 4-chloromethylbenzoyl valerolactam
[0039] Add 0.1 mol of valerolactam into a 500 mL three-neck flask filled with 0.15 mol of triethylamine and 110 mL of toluene, and after being protected by argon, slowly heat to boiling and reflux under magnetic stirring. Slowly add 50 mL of toluene solution dissolved with 0.1 mole of 4-chloromethylbenzoyl chloride into a three-necked flask, condense and reflux for 6 hours under magnetic stirring. After the reaction is complete, cool to room temperature. After filtration, the filtrate was refrigerated overnight. The precipitated white precipitate was filtered off, washed with cold toluene and dried in vacuum for use.
[0040] 2. Dichloro-N,N'-bis(4-benzoylvalerolactammethylene)triethylene diammonium
[0041] Dissolve 0.1 mole of 4-chloromethylbenzoyl valerolactam in 150 mL of acetoni...
Embodiment 3
[0043] This embodiment provides another synthetic method of dicationic bleach activator, the specific steps are as follows:
[0044] 1. Synthesis of 4-chloromethylbenzoyl caprolactam
[0045] Add 0.1 mol of caprolactam into a 500 mL three-necked flask filled with 0.15 mol of triethylamine and 110 mL of toluene, and after being protected by argon, slowly heat to boiling and reflux under magnetic stirring. Slowly add 50 mL of toluene solution dissolved with 0.1 mole of 4-chloromethylbenzoyl chloride into a three-necked flask, condense and reflux for 6 hours under magnetic stirring. After the reaction is complete, cool to room temperature. After filtration, the filtrate was refrigerated overnight. The precipitated white precipitate was filtered off, washed with cold toluene and dried in vacuum for use.
[0046] 2. Dichloro-N,N'-bis(4-benzoylcaprolactammethylene)triethylene diammonium
[0047] Dissolve 0.1 mole of 4-chloromethylbenzoyl valerolactam in 150 mL of acetonitrile, and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| whiteness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com