Silymarin compound and pharmaceutical composition containing extract thereof
A technology of silymarin and compounds, which is applied in the field of silymarin compounds and pharmaceutical compositions containing the extracts, can solve the problems of low dissolution rate, content limitation, and affecting the efficacy of dripping pills, and achieve high dissolution rate and small difference in pill weight , good molding effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
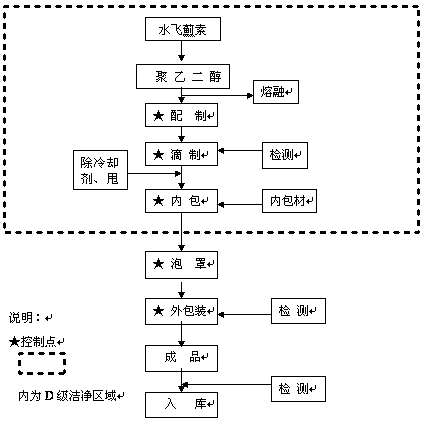
Image
Examples
Embodiment 1
[0028] Prescription: silymarin (calculated as silybin) 7.7g
[0029] Polyethylene glycol 6000 37.3g
[0030] Make 1000 capsules
[0031] Option One
[0032] (1) Heat and melt the prescribed amount of polyethylene glycol 6000 at 80-90°C, and keep stirring at a low speed (10-20r / min). Spray the prescribed amount of silymarin powder that has been ultrafinely pulverized and passed through a 250-mesh sieve. The powder spraying process should be such that the sprayed silymarin powder is evenly sprinkled into the melt to form a uniform eutectic. (2) Raise the temperature of the material to 120°C-130°C, and increase the speed of the mixer to 120-150 rpm for high-speed shearing and stirring for 5-10s until uniform. (3) Reduce the stirring speed to 10-20r / min, and continue to stir for 20-30 minutes to gradually reduce the temperature of the eutectic to 80-90°C. (4) When the temperature of the condensing agent simethicone oil in the dropper of the dropping pill machine an...
experiment example 1
[0050] This experimental example is to study the preparation process of each step of the present invention.
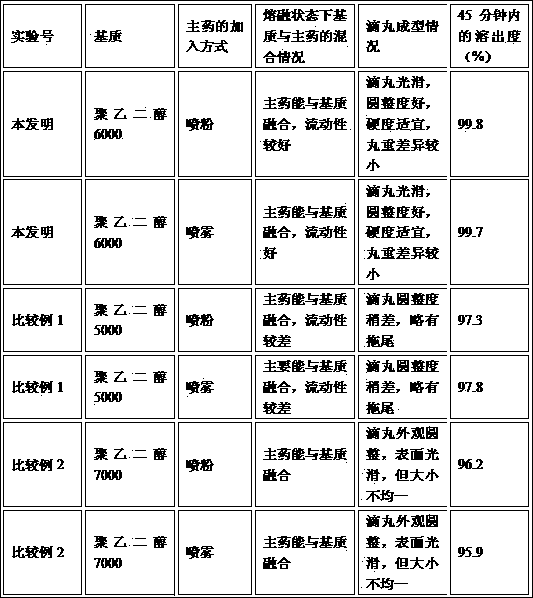
[0051] (1) The influence of the choice of matrix on the drop pills
[0052] In embodiment 1 and comparative example 1 and 2, the dropping pills of different substrates prepared according to the method of the present invention are compared, and the comparison results are shown in Table 1
[0053] Forming comparison of different matrix dropping pills in table 1
[0054]
[0055] It can be seen from the experimental comparison results that the present invention uses polyethylene glycol 6000 as the matrix.
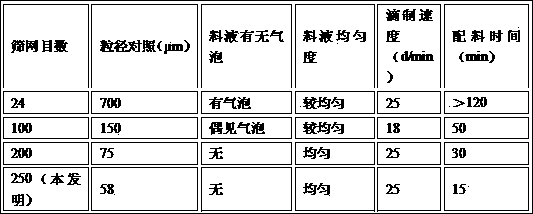
[0056] (2) The influence of the selection of the screen on the dripping pills
[0057] Due to the particularity of the drop pill dosage form, the raw material silymarin needs to be micronized to be evenly dispersed in the matrix. The method of micropowder comprises mechanical pulverization and micropowder crystallization, and what the present invention adopts is ...
Embodiment 2
[0071] This experimental example investigates the dissolution rate of the drop pills made by the preparation process of the present invention, and compares it with the prior art.
[0072] The determination of the dissolution rate of the present invention is based on the 2010 edition of the Chinese Pharmacopoeia Part Two Appendix X C dissolution test method, using artificial gastric juice as the dissolution medium for determination. Wherein the identification amount of dissolution rate is 70%.
[0073] The present invention compares the dissolution rate between commercially available Yiganling tablets, capsules and prior art CN1775208A, CN1490001A drop pills, and compares the dissolution rate of each dosage form within 45min, and the comparison results are as follows:
[0074] The data of table 5 drop pill dissolution rate
[0075]
[0076] The comparison shows that the material ratio and the preparation process adopted in the present invention improve the dissolution r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com