Medicinal composition containing puerarin and preparation thereof
A composition and puerarin technology, applied in the field of medicine, can solve the problems of serious intravascular hemolysis adverse reactions, affecting the therapeutic effect of puerarin, restricting the clinical application of puerarin, etc., achieving easy industrialized production, low production cost, and convenient storage and transportation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
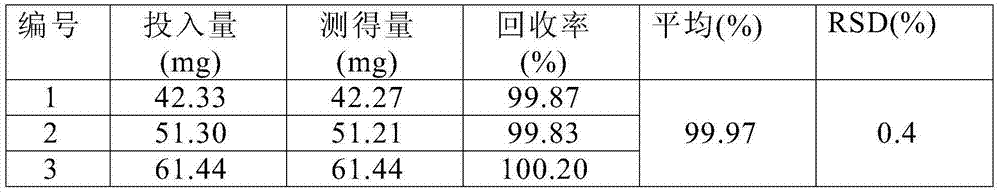
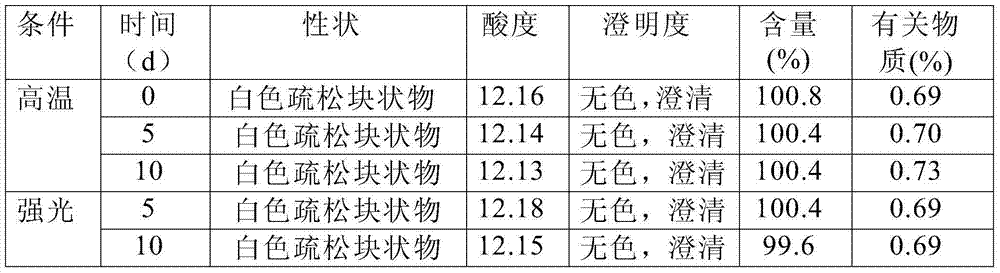
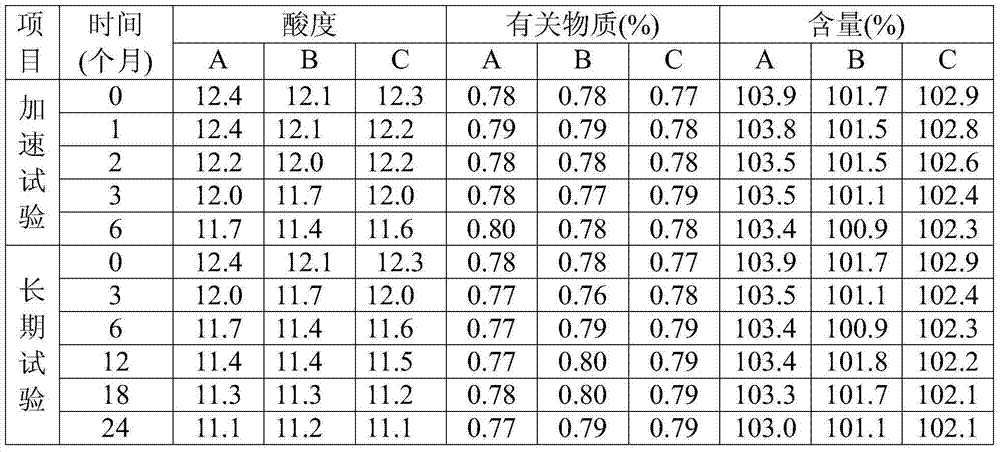
[0069] Under clean conditions, put 5g of glycine into the liquid dispensing device, add 800ml of water for injection and stir to dissolve, cool to room temperature, add sodium hydroxide, adjust the pH to 10.0, blow in nitrogen, add 30g of puerarin and stir for about 30min to make it Completely dissolve, add 40g dextran 60 to dissolve, add 0.3% needle with carbon and stir evenly to absorb the heat source, filter to decarbonize, add water to a sufficient amount, filter through a 0.22μm microporous membrane to obtain a 30mg / ml puerarin solution. Dispense 2.5ml into ampoules, freeze-dry for 72 hours to make sterile freeze-dried powder injection. Specification 30mg / bottle.
Embodiment 2
[0071] Under clean conditions, put 10g of glycine into the liquid dispensing device, add 800ml of water for injection and stir to dissolve, cool to room temperature, add sodium hydroxide, adjust the pH to 10.5, blow in nitrogen, add 30g of puerarin and stir for about 30min to make it Completely dissolve, add 40g dextran 60 to dissolve, add 0.3% needle with carbon and stir evenly to absorb the heat source, filter to decarbonize, add water to a sufficient amount, filter through a 0.22μm microporous membrane to obtain a 30mg / ml puerarin solution. Dispense 2.5ml into ampoules, freeze-dry for 72 hours to make sterile freeze-dried powder injection. Specification 30mg / bottle.
Embodiment 3
[0073] Under clean conditions, put 15g of glycine into the liquid dispensing device, add 800ml of water for injection and stir to dissolve, cool to room temperature, add sodium hydroxide, adjust the pH to 11, feed nitrogen, add 30g of puerarin and stir for about 30min to make it Completely dissolve, add 50g dextran 60 solution, add 0.3% needle with carbon and stir evenly to absorb the heat source, filter to decarbonize, add water to a sufficient amount, filter through a 0.22μm microporous membrane to obtain a 30mg / ml puerarin solution. Dispense 2.5ml into ampoules, freeze-dry for 72 hours to make sterile freeze-dried powder injection. Specification 30mg / bottle.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com