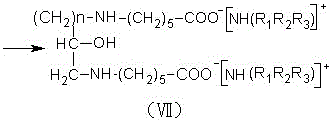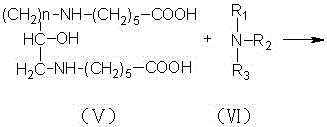A kind of synthetic method of hydroxyalkyl tertiary amine of beta-hydroxyalkanediaminocaproic acid
A technology of diaminocaproic acid hydroxyalkyl tertiary amine and diaminocaproic acid sodium, which is applied to the preparation of amino hydroxyl compounds, chemical instruments and methods, and the preparation of organic compounds, etc., and can solve the problems of few product varieties and limited application coverage, etc. problems, achieve strong anti-rust effect, improve anodic polarization performance, and inhibit corrosion effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
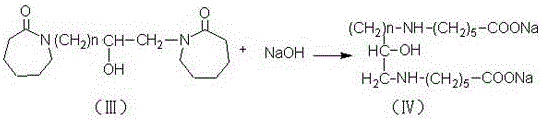
[0023] In a four-necked flask equipped with a stirring device, a reflux condensing device and a thermometer, add 159 grams of benzene, add 2 grams of zinc chloride, 1 gram of zinc oxide and 106 grams of caprolactam, and slowly add 29 grams of chlorinated epoxy Propane, add saturated aqueous solution of sodium carbonate to the reaction mixture, raise the temperature to 80°C~90°C and continue the reflux reaction for 7 hours. During the reaction, use an acid binding agent to control the pH at 8.5~9.5 to generate biscaprolactam-2-ol, and the reaction is completed Afterwards, cool to 30°C, stop stirring, add 120 grams of 47% sodium hydroxide solution to the reaction mixture to dilute and hydrolyze the reaction, the reaction mixture is divided into two layers, the upper layer is an organic phase, and the lower layer is separated to obtain a liquid β-hydroxyalkane Diethylene diaminocaproic acid sodium solution, slowly add 30% sulfuric acid solution to the β-hydroxyalkylene diaminocapr...
Embodiment 2
[0025] In a four-neck flask equipped with a stirring device, a reflux condensing device and a thermometer, add 165 grams of benzene, 2 grams of zinc chloride, 2 grams of zinc oxide and 82.5 grams of caprolactam, and slowly add 31 grams of chlorinated epoxy Butane, add saturated aqueous solution of sodium carbonate to the reaction mixture, raise the temperature to 85°C-95°C and continue the reflux reaction for 6 hours. During the reaction, use an acid-binding agent to control the pH at 8.5-9.5 to generate biscaprolactam-2-ol. After completion, cool to 40°C, stop stirring, add 100 grams of 44% sodium hydroxide solution to the reaction mixture for dilution and hydrolysis reaction, the reaction mixture is divided into two layers, the upper layer is the organic phase, and the lower layer is separated to obtain liquid β-hydroxyl Sodium alkylene diaminocaproate solution, slowly add 20% hydrochloric acid to the sodium β-hydroxyalkylene diaminocaproate solution, adjust the pH to 1.0, an...
Embodiment 3
[0027] In a four-neck flask equipped with a stirring device, a reflux condensing device and a thermometer, add 176 grams of benzene, 1.5 grams of zinc chloride, 1.2 grams of zinc oxide and 70.5 grams of caprolactam, and slowly add 33 grams of chlorinated epoxy Butane, add saturated aqueous solution of sodium carbonate to the reaction mixture, raise the temperature to 90°C-95°C and continue the reflux reaction for 8 hours. During the reaction, use an acid-binding agent to control the pH at 8.5-9.5 to generate biscaprolactam-2-ol. After completion, cool to 20°C, stop stirring, add 95 grams of 40% sodium hydroxide solution to the reaction mixture for dilution and hydrolysis reaction, the reaction mixture is divided into two layers, the upper layer is an organic phase, and the lower layer is separated to obtain liquid β-hydroxyl Sodium alkylene diaminocaproate solution, slowly add 35% sulfuric acid solution to the sodium β-hydroxyalkylene diaminocaproate solution, adjust the pH to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com