Novel photosensitizer monomer completely soluble in water, and preparation method and application thereof
A technology of monomer and water-soluble salt, applied in the field of completely water-soluble photosensitizer monomer and its preparation, which can solve problems such as complicated process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
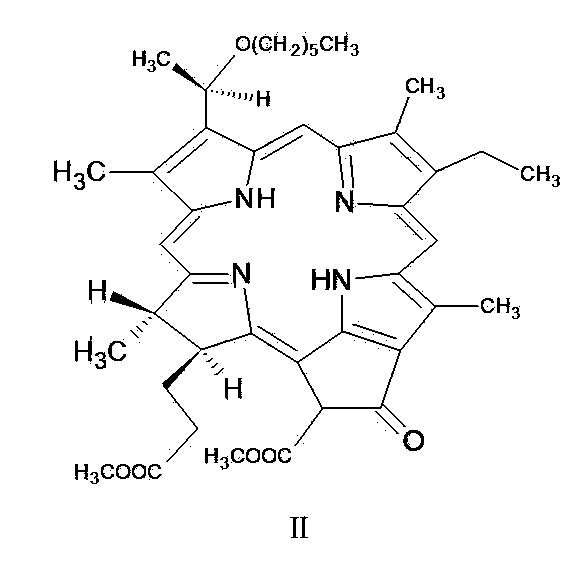
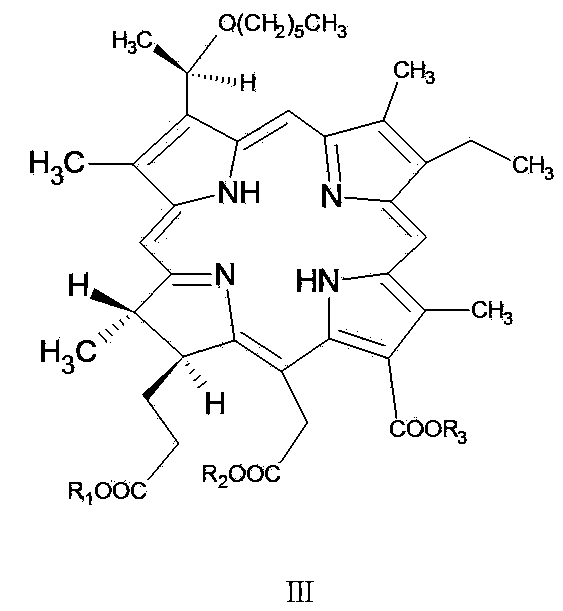
[0060] One, the preparation of pheophorbide-a methyl ester (MPPB)
[0061] Chlorophyll-a extract (100g) is dissolved in pre-prepared volume ratio concentration of H 2 SO 4 5% H 2 SO 4 / methanol mixed solution, the reaction mixture was stirred at room temperature for 4h, and after the raw materials and intermediate products were completely reacted, it was poured into water and washed with CH 2 Cl 2 After extraction, the organic layer was washed with water, and the residue after distilling off the solvent under reduced pressure was crystallized with ethyl acetate / vegetable oil extraction solvent (volume ratio: 1 / 1).
[0062] 2. Preparation and crude production of 3-desvinyl-3-(1'-hexylhydroxy)ethyl-pheophorbide-a methyl ester
[0063] Dissolve 50 g of pheophorbide-a methyl ester in CH 2 Cl 2 (350ml), add 30% hydrogen bromide / acetic acid solution (300ml), the reaction mixture is stirred at about 20°C for 3-5h, then the solvent is evaporated in vacuo (water bath temperature...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com