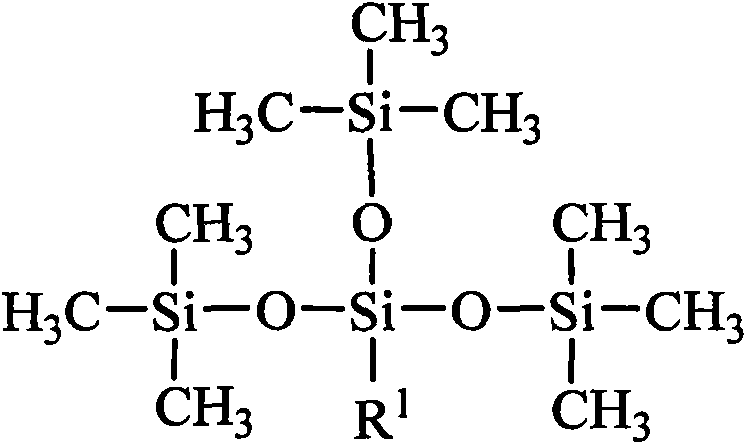
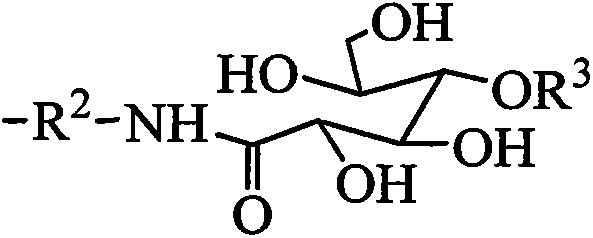
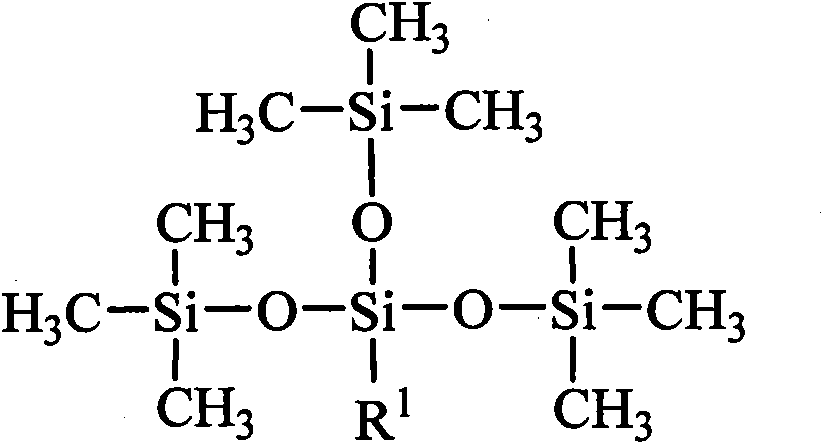
Tetrasiloxane containing sugar acylamino and preparation method
A technology containing sugar amide groups and tetrasiloxane, which is applied in the field of organosilicon compound preparation, can solve the problems of affecting performance and losing surface activity, and achieve the effect of good adsorption performance and difficult hydrolysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Add 1.62kg of hexamethyldisiloxane, 1.99kg of chloropropyltrimethoxysilane, and 0.006kg of concentrated sulfuric acid into reaction kettle 1, react at a temperature of 40°C for 4 hours, add sodium hydroxide to deactivate the catalyst, and filter The solid was removed and distilled under reduced pressure to obtain chloropropyltetrasiloxane. Add 3.73kg of chloropropyltetrasiloxane and 0.74kg of 1,3-propylenediamine into the reaction kettle 2, heat to 60°C, and react for 4 hours. - Aminopropyl-γ-aminopropyltetrasiloxane. Add 4.10kg of N-β-aminopropyl-γ-aminopropyltetrasiloxane and 1.78kg of gluconolactone into reaction kettle 3, use methanol as a solvent, heat to 60°C, react for 8 hours, and evaporate the solvent methanol to obtain tetrasiloxane containing sugar amido groups. It is measured that it does not hydrolyze within 30 days in water.
Embodiment 2
[0030] Add 8.12kg of hexamethyldisiloxane, 1.99kg of chloropropyltrimethoxysilane, and 0.10kg of acid clay to reaction kettle 1, react at a temperature of 50°C for 4 hours, remove the catalyst by filtration, and distill under reduced pressure to obtain chloropropane Tetrasiloxane. Add 3.73kg of chloropropyltetrasiloxane and 2.64kg of 1,4-butanediamine into the reaction kettle 2, heat to 80°C, and react for 3 hours. - Aminobutyl-γ-aminopropyltetrasiloxane. Add 4.24kg of N-β-aminobutyl-γ-aminopropyltetrasiloxane and 1.96kg of gluconic acid into the reaction kettle 3, use ethanol as solvent, heat to 70°C, react for 8 hours, evaporate the solvent ethanol, Tetrasiloxane containing sugar amido groups is obtained. It is measured that it does not hydrolyze within 30 days in water.
Embodiment 3
[0032] Add 16.24kg of hexamethyldisiloxane, 1.99kg of chloropropyltrimethoxysilane, and 0.54kg of concentrated sulfuric acid into reaction kettle 1, react at a temperature of 60°C for 4 hours, add sodium hydroxide to deactivate the catalyst, and filter The solid was removed and distilled under reduced pressure to obtain chloropropyltetrasiloxane. Add 3.73kg of chloropropyltetrasiloxane and 6.12kg of 1,5-pentamethylenediamine into the reaction kettle 2, heat to 90°C, react for 2 hours, the mixture is allowed to stand for layers, and the upper layer is distilled under reduced pressure to obtain N-β -Aminoamyl-γ-aminopropyltetrasiloxane. Add 4.38kg of N-β-aminopentyl-γ-aminopropyltetrasiloxane and 1.78kg of mannolactone to reaction kettle 3, use methanol as solvent, heat to 80°C, react for 10 hours, evaporate Solvent methanol to obtain tetrasiloxane containing sugar amido group. It is measured that it does not hydrolyze within 30 days in water.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com