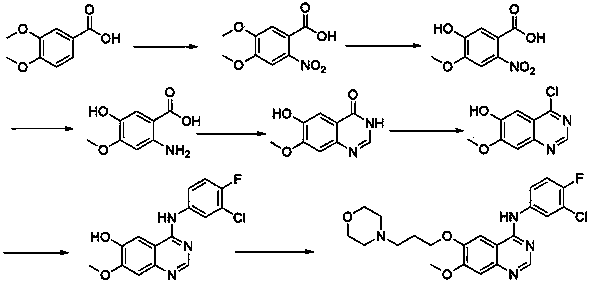
Preparing method of anticancer medicine
An anticancer drug, gefitinib technology, applied in the field of medicinal chemistry, can solve the problems of many by-products, low yield, many side reactions, etc., and achieve the effects of less three wastes, high yield, and reducing the generation of side reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
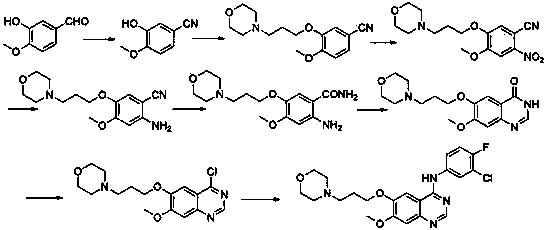
[0039] The first step: 2-amino-4-methoxy-5-(3-morpholine propoxy) benzonitrile hydrochloride ( 3 ) preparation
[0040] Put 100.00 g of 4-methoxy-5-(3-morpholine propoxy)-2-nitrobenzonitrile into a reaction flask containing 300 ml of dioxane and 1000 ml of water, stir and heat to 70°C , then slowly add 325.20g of sodium hydrosulfite, keep warm for 6h, add 100mL of concentrated hydrochloric acid dropwise, after the addition is complete, the solution is clear. Cool the reaction solution to room temperature, add 120g of sodium hydroxide solution (30%), adjust the pH to strong alkalinity, extract with dichloromethane (1000ml×3), combine the dichloromethane phase, wash the dichloromethane phase with water, Then washed with saturated brine, anhydrous Na 2 SO 4 Drying, filtration, and concentration gave crude 2-amino-4-methoxy-5-(3-morpholinopropoxy)benzonitrile. The above crude product was dissolved in 1000ml of absolute ethanol, and concentrated hydrochloric acid was added drop...
example 2
[0042] The first step: 2-amino-4-methoxy-5-(3-morpholine propoxy) benzonitrile hydrochloride ( 3 ) preparation
[0043] Put 100.00 g of 4-methoxy-5-(3-morpholine propoxy)-2-nitrobenzonitrile into a reaction flask filled with 1000 ml of dioxane and 300 ml of water, stir and heat to 50°C , and then slowly add 162.67g of sodium hydrosulfite, keep warm for 5h, add 100mL of concentrated hydrochloric acid dropwise, after the dropwise addition, the solution is clear. Cool the reaction solution to room temperature, add 120g of sodium hydroxide solution (40%), adjust the pH to strong alkalinity, extract with dichloromethane (800ml×3), combine the dichloromethane phase, wash the dichloromethane phase with water, Then washed with saturated brine, anhydrous Na 2 SO 4 Drying, filtration, and concentration gave crude 2-amino-4-methoxy-5-(3-morpholinopropoxy)benzonitrile. The above crude product was dissolved in 700ml of absolute ethanol, and concentrated hydrochloric acid was added drop...
example 3
[0045] Step two: N '-(2-cyano-5-methoxy-4-(3-morpholinopropoxy)phenyl)- N , N -Dimethylformamide imine ( 4 ) preparation
[0046] the intermediate ( 3 ) 80.00g and N,N-dimethylformamide dimethyl acetal 169.00g were added to 1000ml toluene, heated to 100°C for 2h, and the reaction was stopped. Concentrate under reduced pressure to obtain a viscous liquid, add the obtained viscous liquid to 280ml of water, extract with dichloromethane (280ml×3), combine the dichloromethane phases, wash with water, wash with saturated brine, dry over anhydrous sodium sulfate, and filter , concentrated to obtain the crude product. Add 260ml of absolute ethanol to the above crude product, heat to reflux until the crude product is completely dissolved, cool to 10°C to crystallize, filter, and collect 66.30g of solid. Yield 78%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com