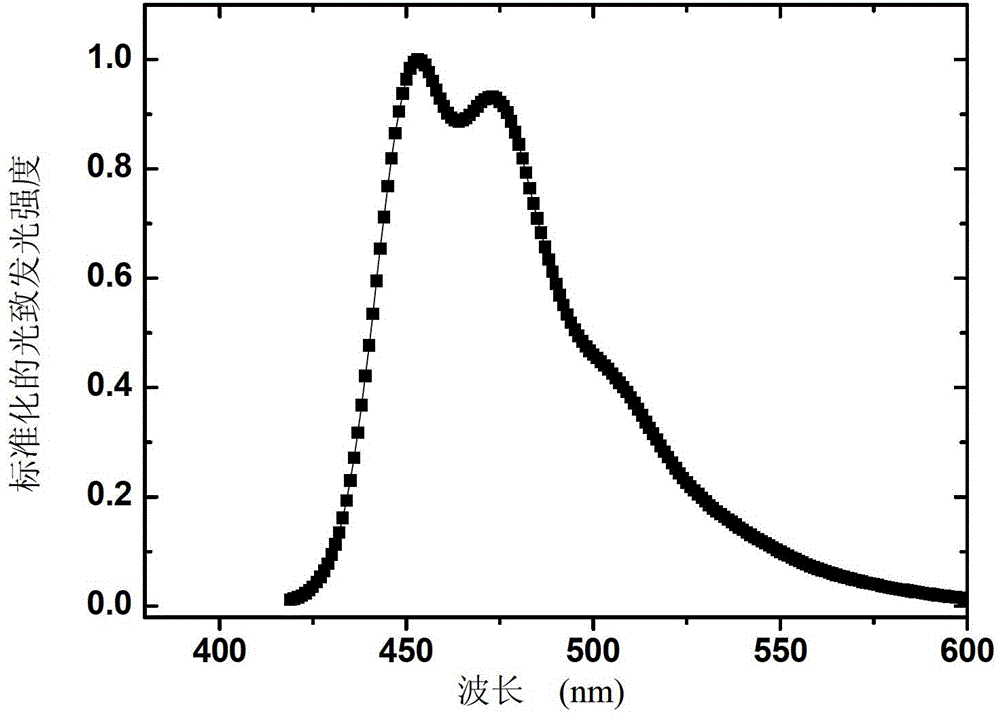
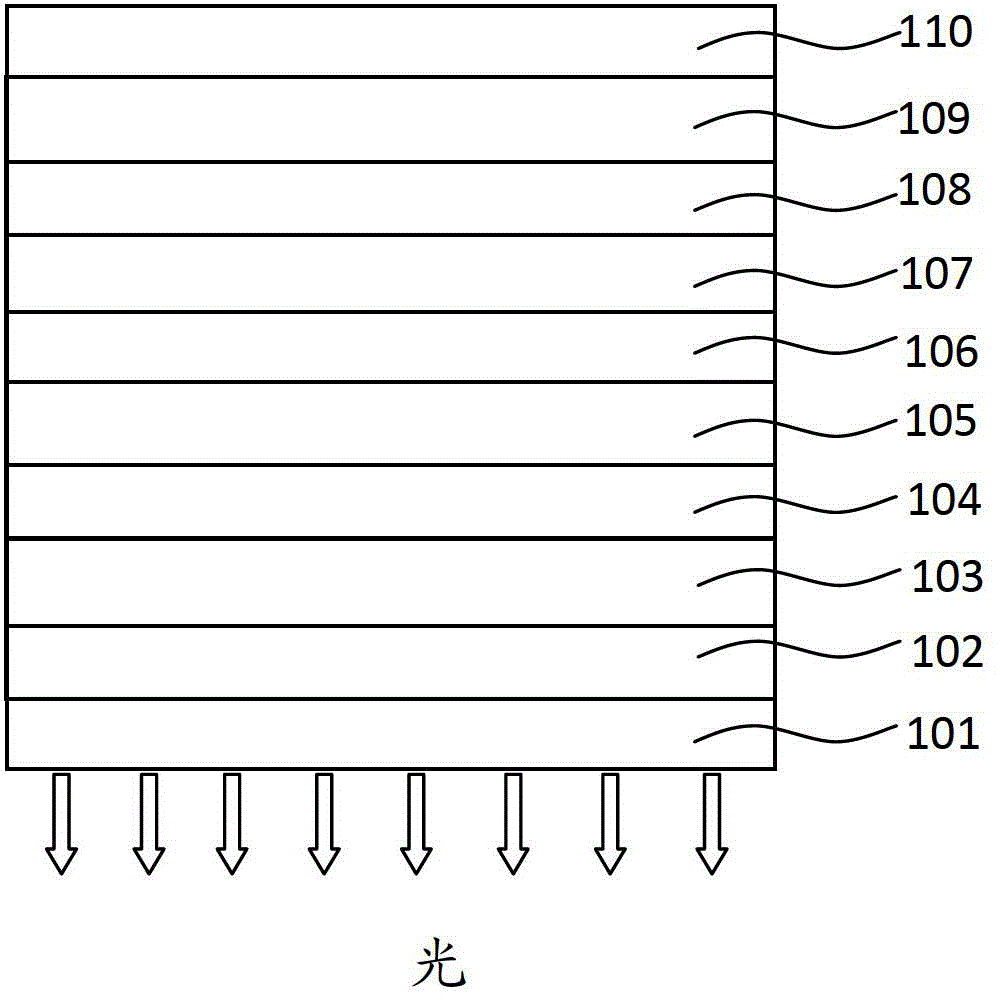
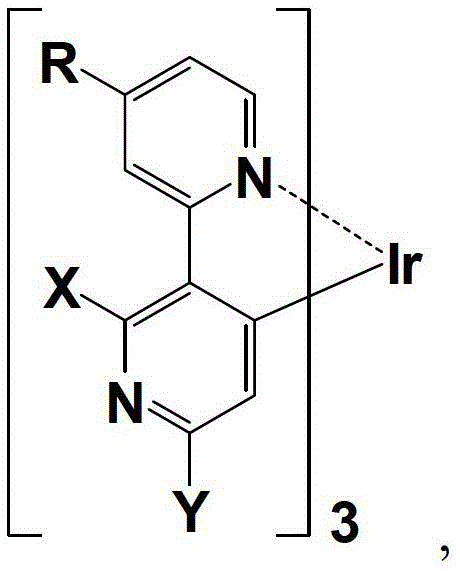
Organic phosphorescence material and preparation method thereof and organic electroluminescent device
A technology of optical materials and organophosphorus, applied in luminescent materials, electrical solid devices, organic chemistry, etc., can solve the problems of luminous color purity, luminous efficiency, insufficient attenuation of device efficiency, short life, poor stability, etc., and achieve good energy Effects of transmission efficiency, improvement of luminous performance, and low processing cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1: An organic phosphorescent material complex tris(4-methyl-2',6'-dichloro-2,3'-bipyridine-N,C 4 ') The preparation method of iridium organic phosphorescent material, comprises the steps:
[0048] (1) Provide compound C1 and compound D1 represented by the following structural formula respectively:
[0049]
[0050] (2) Synthesis of 2,6-dichloro-4'-methyl-3,2'-bipyridine
[0051]
[0052] Under nitrogen protection, (1.03g, 6.00mmol) 2-bromo-4-picoline, (1.38g, 7.20mmol) 2,6-dichloro-3-pyridineboronic acid, (3.04g, 22mmol) K 2 CO 3 and (0.35g,0.30mmol)Pd(PPh 3 ) 4 Dissolve in a mixed solution of 30mL toluene and 5mL water, heat to reflux, and stir for 20h. After cooling to room temperature, the aqueous phase in the reaction mixture was separated and extracted three times with 100 mL of ethyl acetate, and the organic phases were combined. The combined organic phases were washed with saturated brine and dried over anhydrous magnesium sulfate. After filtra...
Embodiment 2
[0061] Example 2: An organic phosphorescent material complex tris(4-tert-butyl-2',6'-dichloro-2,3'-bipyridine-N,C 4 ') The preparation method of iridium organic phosphorescent material, comprises the steps:
[0062] (1) Provide compound C2 and compound D2 represented by the following structural formula respectively:
[0063]
[0064] (2) Synthesis of 2,6-dichloro-4'-tert-butyl-3,2'-bipyridine
[0065]
[0066] Under nitrogen protection, (1.28g, 6.00mmol) 2-bromo-4-tert-butylpyridine, (1.73g, 9.00mmol) 2,6-dichloro-3-pyridineboronic acid, (0.48g, 12mmol) NaOH and (0.07g,0.06mmol)Pd(PPh 3 ) 4 Dissolve in a mixed solution of 60mL toluene and 5mL water, heat to reflux, and stir for 10h. After cooling to room temperature, the aqueous phase in the reaction mixture was separated and extracted three times with 100 mL of ethyl acetate, and the organic phases were combined. The combined organic phases were washed with saturated brine and dried over anhydrous magnesium sulfate...
Embodiment 3
[0074] Example 3: An organic phosphorescent material complex tris(4-n-hexyl-2',6'-dichloro-2,3'-bipyridine-N,C 4 ') The preparation method of iridium organic phosphorescent material, comprises the steps:
[0075] (1) Provide compound C3 and compound D3 represented by the following structural formulas respectively:
[0076]
[0077] (2) Synthesis of 2,6-dichloro-4'-n-hexyl-3,2'-bipyridine
[0078]
[0079] Under nitrogen protection, (1.45g, 6.00mmol) 2-bromo-4-tert-butylpyridine, (1.73g, 9.00mmol) 2,6-dichloro-3-pyridineboronic acid, (0.48g, 12mmol) NaOH and (0.07g,0.06mmol)Pd(PPh 3 ) 4 Dissolve in a mixed solution of 60mL DMF and 5mL water, heat to reflux, and stir for 10h. After cooling to room temperature, the aqueous phase in the reaction mixture was separated and extracted three times with 100 mL of ethyl acetate, and the organic phases were combined. The combined organic phases were washed with saturated brine and dried over anhydrous magnesium sulfate. After ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com