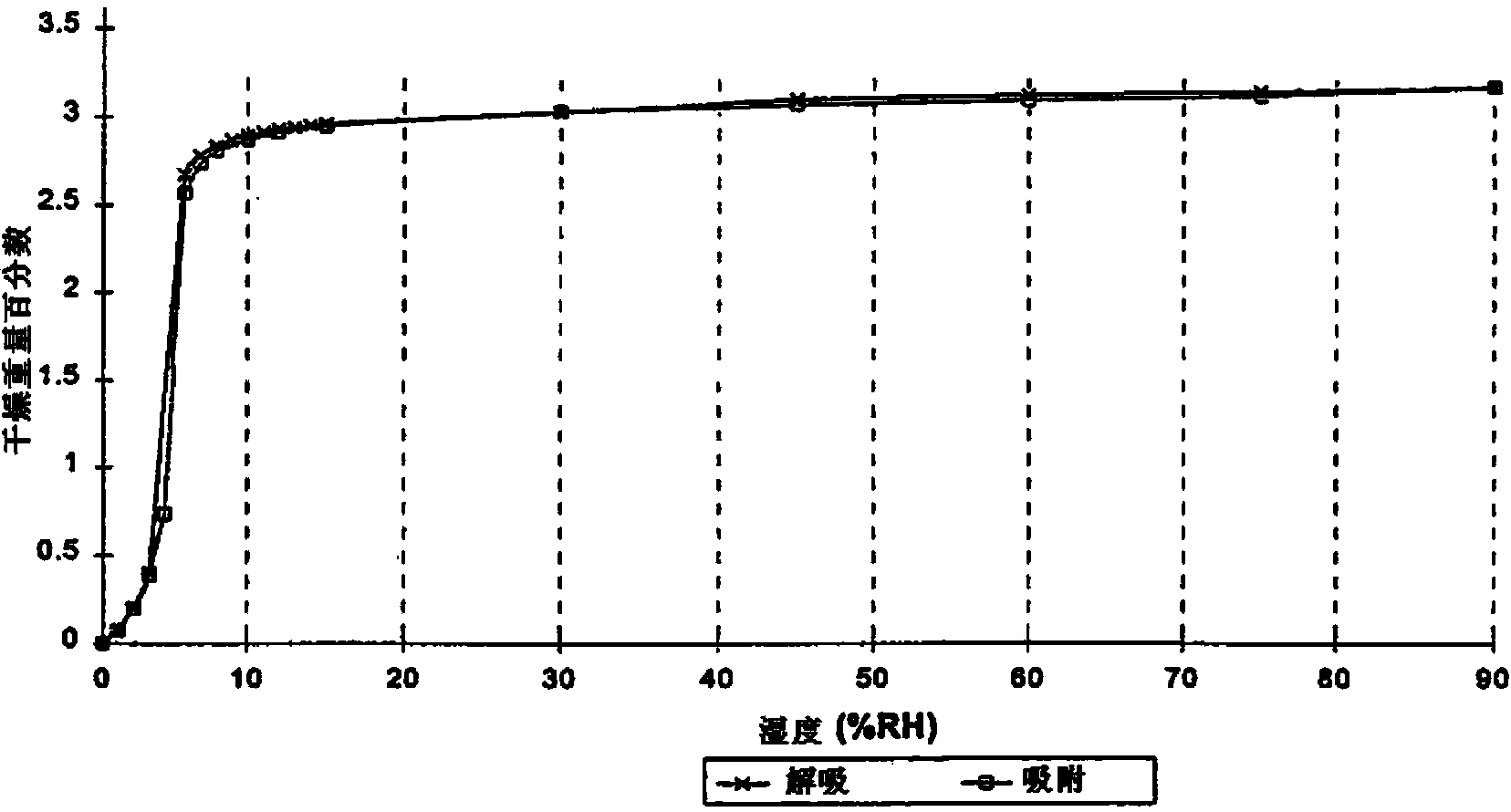
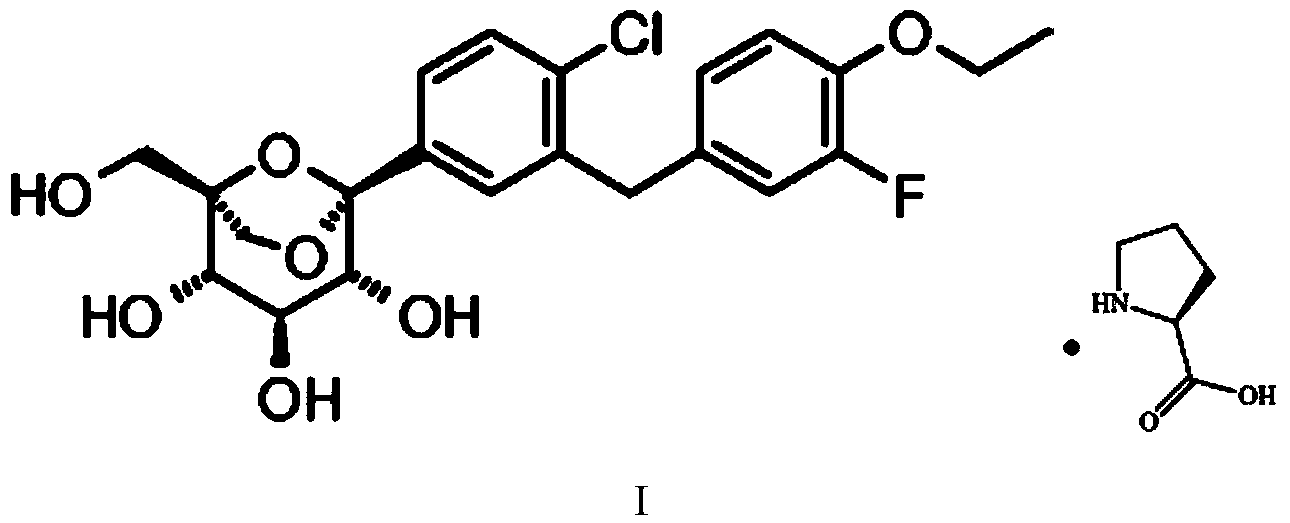
Hypoglycemic medicine
A drug and pharmaceutical technology, applied in the field of sodium-dependent glucose transporter inhibitors, can solve problems such as lactic acidosis, unsatisfactory long-term treatment, easy-induced edema and heart failure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0122] Preparation example 1: Preparation of 1-ethoxy-2-fluoro-benzene
[0123]
[0124] 2-Fluorophenol (6.7g, 60mmol) was dissolved in 66mL of acetone, iodoethane (6.3mL, 78mmol) and potassium carbonate (12.4g, 90mmol) were added, and the mixture was refluxed in an oil bath for 5 hours. Concentrate the reaction solution under reduced pressure, add 100mL ethyl acetate and 60mL water, separate the layers, extract the aqueous phase with ethyl acetate (30mL×2), combine the organic phases, dry over anhydrous magnesium sulfate, filter, and concentrate the filtrate under reduced pressure to obtain the title Product 1-ethoxy-2-fluoro-benzene (6.88 g, red oil). MS m / z(ESI): 280.2[2M+1]
preparation example 2
[0125] Preparation example 2:Preparation of (5-bromo-2-chloro-phenyl)-(4-ethoxy-3-fluoro-phenyl)methanone
[0126]
[0127] Dissolve 5-bromo-2-chloro-benzoyl chloride (12.4g, 48.8mmol) in 100mL of dichloromethane, add 1-ethoxy-2-fluoro-benzene (6.84g, 48.8mmol), cool to 0 °C, aluminum trichloride (5.86 g, 44 mmol) was added in batches, and reacted for 16 hours. Add 20 mL of 2M hydrochloric acid solution dropwise to the reaction solution under ice bath, separate the layers, extract the aqueous phase with 30 mL of dichloromethane, combine the organic phases, dry over anhydrous magnesium sulfate, filter, and concentrate the filtrate under reduced pressure to obtain the title product (5-bromo -2-Chloro-phenyl)-(4-ethoxy-3-fluoro-phenyl)methanone (12.8 g, yellow solid). MS m / z (ESI): 358.9 [M+1].
preparation example 3
[0128] Preparation example 3: Preparation of (5-bromo-2-chloro-phenyl)-(4-ethoxy-3-fluoro-phenyl)methanol
[0129]
[0130] (5-Bromo-2-chloro-phenyl)-(4-ethoxy-3-fluoro-phenyl)methanone (12.7 g, 35.5 mmol) was dissolved in 100 mL THF and methanol (v:v=1: To the mixed solvent of 1), sodium borohydride (2.68 g, 70 mmol) was added in batches under ice-cooling, and the reaction was carried out at room temperature for 30 minutes. Add 15 mL of acetone, concentrate the reaction solution under reduced pressure, add 150 mL of ethyl acetate to dissolve the residue, wash with saturated sodium chloride solution (50 mL×2), combine the organic phases, dry over anhydrous magnesium sulfate, filter, and concentrate the filtrate under reduced pressure to obtain The title product (5-bromo-2-chloro-phenyl)-(4-ethoxy-3-fluoro-phenyl)methanol (12.65 g, orange oil) was used in the next reaction without isolation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com