Detection method for relative substances in compound aminophenazone and barbital injection
A compound ammonium barbital and detection method technology, applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve problems such as difficulties, establishment of substance detection methods, and loss of chromatographic columns, and achieve the goals of wide application range and drug safety guarantee Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Embodiment 1 Detection method of the present invention
[0067] Instruments and conditions: Agilent1200 liquid chromatograph, DAD detector, chromatographic column: AgilentTC-C18 (250mm*4.6mm, 5um); detection wavelength: 220nm; column temperature: 30°C; buffered with 0.02mol / L potassium dihydrogen phosphate solution (pH adjusted to 5.5) - acetonitrile = 85:15 as mobile phase A, acetonitrile as mobile phase B, perform linear gradient elution in the following table.
[0068] time / minute
Mobile phase A / %
Acetonitrile / %
0
100
0
30
100
0
50
50
50
55
50
50
56
100
0
65
100
0
[0069] main peak positioning
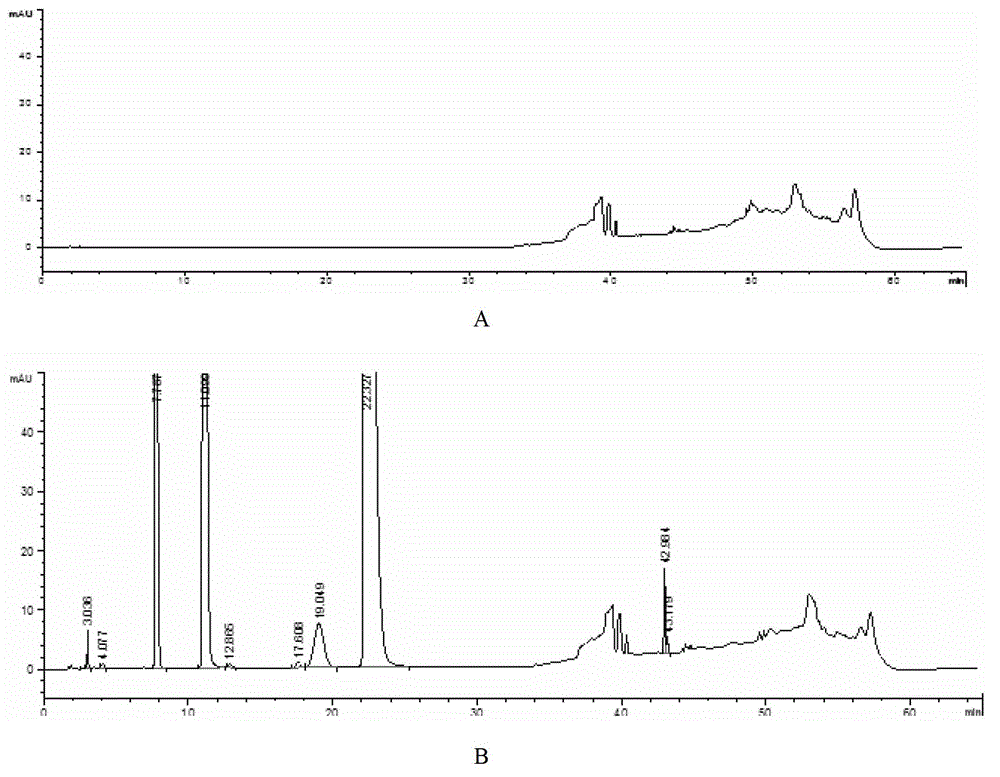
[0070] Take appropriate amount of reference substances of aminopyrine, antipyrine and barbiturate respectively, add mobile phase A to prepare solutions with concentrations of 0.05mg / ml, 0.02mg / ml, and 0.009mg / ml. Take another 1ml of compound ammonium barbiturate ...
Embodiment 2
[0076] Embodiment 2 detection method of the present invention
[0077] Instruments and conditions: Agilent1200 liquid chromatograph, DAD detector, chromatographic column: AgilentTC-C18 (250mm*4.6mm, 5um); detection wavelength: 220nm; column temperature: 30°C; buffered with 0.02mol / L potassium dihydrogen phosphate solution (pH adjusted to 6.0)-acetonitrile=85:15 as mobile phase A, acetonitrile as mobile phase B, and perform linear gradient elution in the following table.
[0078] time / minute
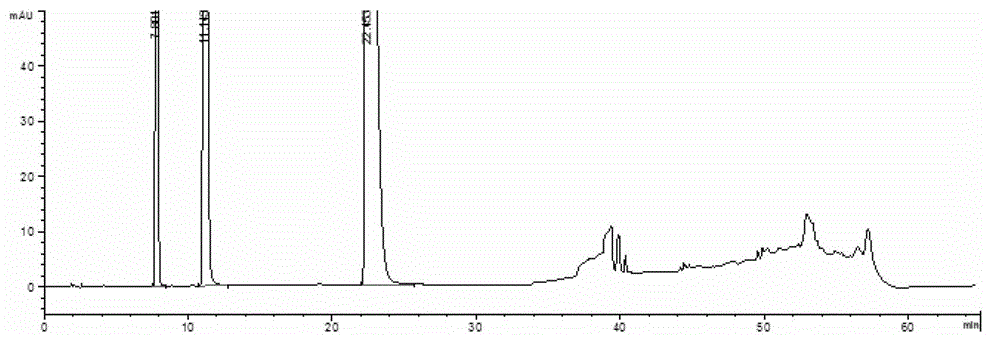
[0079] Take 1ml of compound ammonium barbiturate injection and put it in a 100ml measuring bottle, after diluting and constant volume with mobile phase A, detect according to the above method, the HPLC spectrum is shown in image 3 .
Embodiment 3
[0080] Embodiment 3 Determination of Related Substances of Compound Ammonium Barbital Injection
[0081] Instruments and conditions: Agilent1200 liquid chromatograph, DAD detector, chromatographic column: Agilent TC-C18 (250mm*4.6mm, 5um); detection wavelength: 220nm; column temperature: 30°C; with 0.02mol / L potassium dihydrogen phosphate Buffer solution (pH adjusted to 5.5)-acetonitrile=83:17 as mobile phase A, acetonitrile as mobile phase B, perform linear gradient elution in the following table.
[0082] time / minute
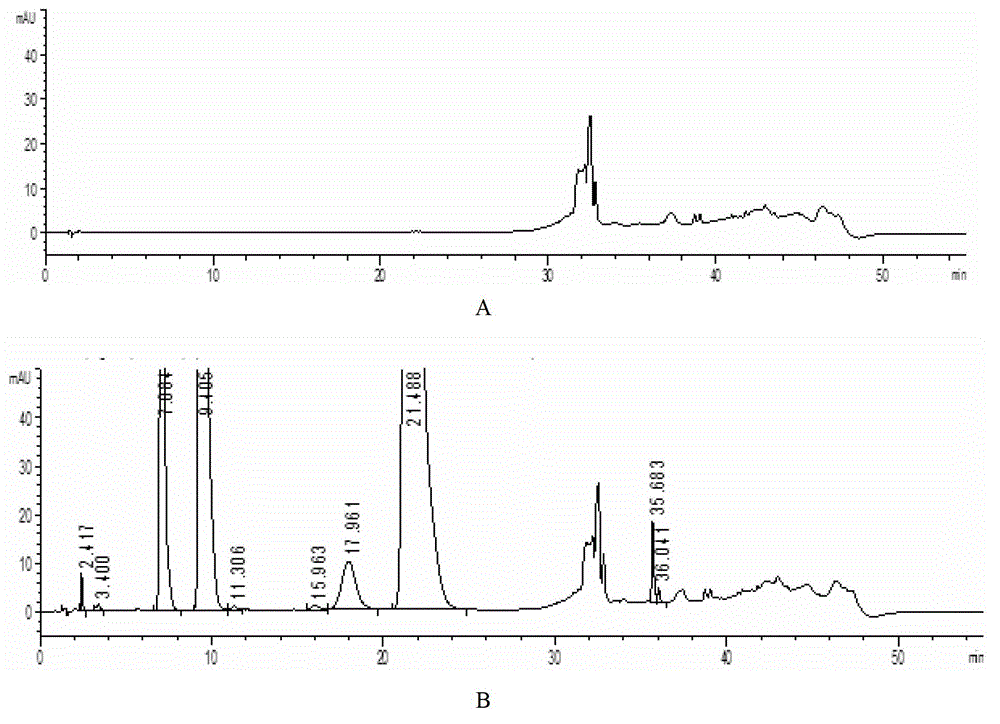
[0083] Experimental procedure: Measure 1ml of the long-term 36-month sample of compound ammonium barbiturate injection preparation and place it in a 100ml measuring bottle, add mobile phase A to constant volume and shake up, measure according to the law, see HPLC spectrum Figure 4 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com