Application of Antigen Containing 6-n-Methyllysine Residue in Preparation of Reagent for Auxiliary Diagnosis of Systemic Lupus Erythematosus
A technology of methyllysine and 6-N-, applied in the field of application of antigens containing 6-N-methyllysine residues in the preparation of reagents for auxiliary diagnosis of systemic lupus erythematosus, which can solve the problem And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1. Use H3 1-19 K4me-BSA and H3 1-19 K9me-BSA Auxiliary Diagnosis of SLE
[0032] 1. Preparation of peptides and their peptide-BSA crosslinks
[0033] This step produces polypeptide H3 containing 6-N-methyllysine residues 1-19 K4me and H3 1-19 K9me. H3 1-19 K4me and H3 1-19 K9me has the same amino acid sequence but different lysine methylation modification positions, H3 1-19 K4me is the 4th lysine methylation modification, H3 1-19 K9me is methylation modification of lysine 9.
[0034] The above-mentioned peptides were synthesized by Beijing Zhongke Yaguang Co., Ltd. by solid-phase peptide synthesis method, with a purity of ≧90%. Its sequence is shown in Table 1. Each polypeptide end contains a cysteine residue, and is cross-linked with BSA through this cysteine residue to form a cross-linker, namely H3 1-19 K4me-BSA and H3 1-19 K9me-BSA. The cross-linking method is as follows: 2mgBSA is dissolved in the reaction solution (0.1MNaH 2 PO 4 , Na...
Embodiment 2
[0050] Example 2. Anti-H3 1-19 The epitope of the K9meIgM antibody is a monomethylated lysine
[0051] 1. Preparation of polypeptide and its polypeptide-BSA cross-linked product
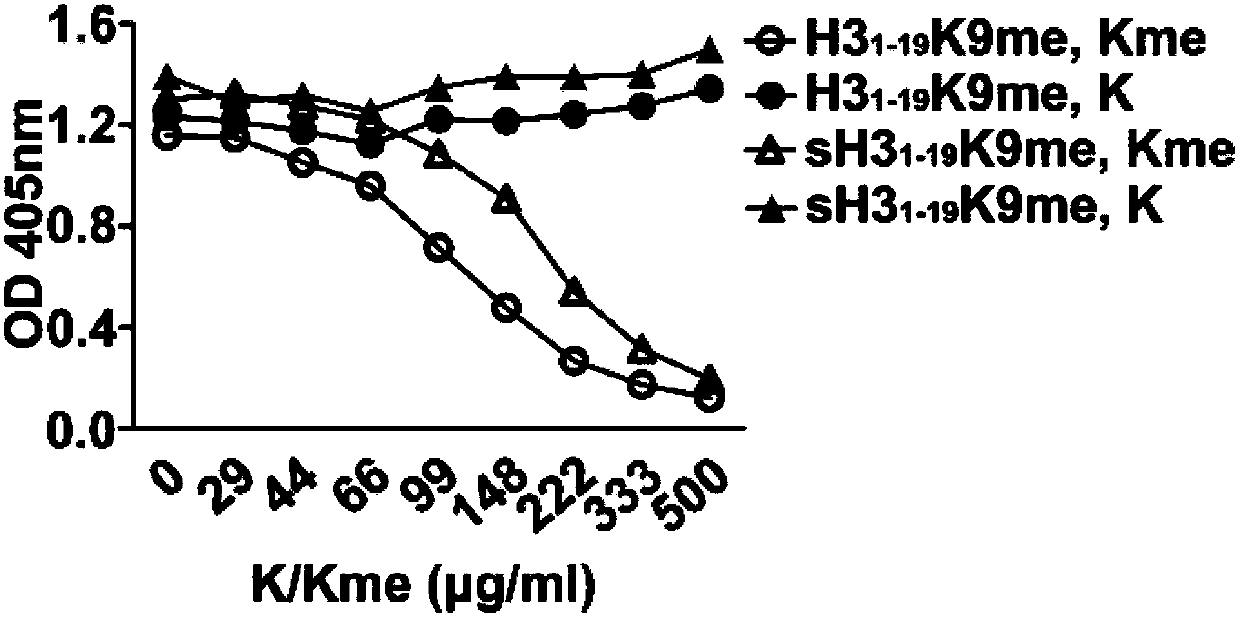
[0052] This step prepared 11 polypeptides listed in Table 1, namely GGKme, sH3 1-19 K9me, H3 1-19 K9me, H3 1-19 K4me, H31-19 , H3 1-19 K4me2, H3 1-19 K4me3, H3 1-19 K9me2, H3 1-19 K9me3 and H3 1-19 K9ac. The number after K indicates the position of lysine in the polypeptide, me indicates monomethylation modification, me2 indicates dimethylation modification, me3 indicates trimethylation modification, and ac indicates acetylation modification. where H3 1-19 , H3 1-19 K4me, H3 1-19 K4me2, H3 1-19 K4me3, H3 1-19 K4me, H3 1- 19 K9me2, H3 1-19 K9me3 and H3 1-19 K9ac has the same amino acid sequence but H3 1-19 No modification, other lysine modifications vary in position. H3 1-19 K9me and H3 1-19 The composition of K9me is exactly the same, but the amino acid sequence is completely di...
Embodiment 3
[0065] Example 3. Assisted diagnosis of SLE with GGKme-BSA
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com