Catalyst for preparing methacrylic acid by oxidation of methylacrolein and preparation method of catalyst
A technology of methacrylic acid and methacrolein, which is applied in the preparation of organic compounds, catalysts for physical/chemical processes, and preparation of carboxylate, which can solve the problems of short catalytic life and low space-time yield of products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] 1KgMoO 3 , V 2 o 5 57.4g, 17%H 3 PO 4 364.1 g was added into 15 L of deionized water, stirred, the temperature was raised to 105°C, stirred, condensed and refluxed for about 3 hours until the solution became orange-red solution A. Unreacted solids were filtered off. Take 1 / 3 of filtrate A, namely 5.1L, add CsNO 3 164.1 g, stirred vigorously at room temperature for 2 hours, centrifuged after the reaction was complete, poured out the supernatant, solid precipitate A, washed with deionized water.
[0029] Add the precipitate A to 1 / 3 of the solution A, slowly add 129.6ml of 25% ammonia water dropwise under vigorous stirring, and continue stirring for 2h after the dropwise addition is completed. The resulting solution was then centrifuged to obtain solid precipitate B, which was washed with deionized water.
[0030] Add the precipitate B to the remaining 1 / 3 solution A, raise the temperature to 85°C while stirring, and add 59.4g Cu(NO 3 ) 2 , 25.5gFe(NO 3 ) 3 , 1...
Embodiment 2
[0035] The preparation method of Solution A, Precipitation A and Precipitation B is as in Example 1. Precipitation B is added to the remaining 1 / 3 Solution A, and the temperature is raised to 85°C while stirring, and 59.4g Cu (NO 3 ) 2 , 25.5gFe(NO 3 ) 3 , 38.3gMn(NO 3 ) 4 and 2.5gTiO 2 Add 1.2 L of deionized water to make a solution, slowly drop it into the suspension, react for 2 hours after the dropwise addition, concentrate and dry, and finally obtain the catalyst precursor B. Its composition expression is:
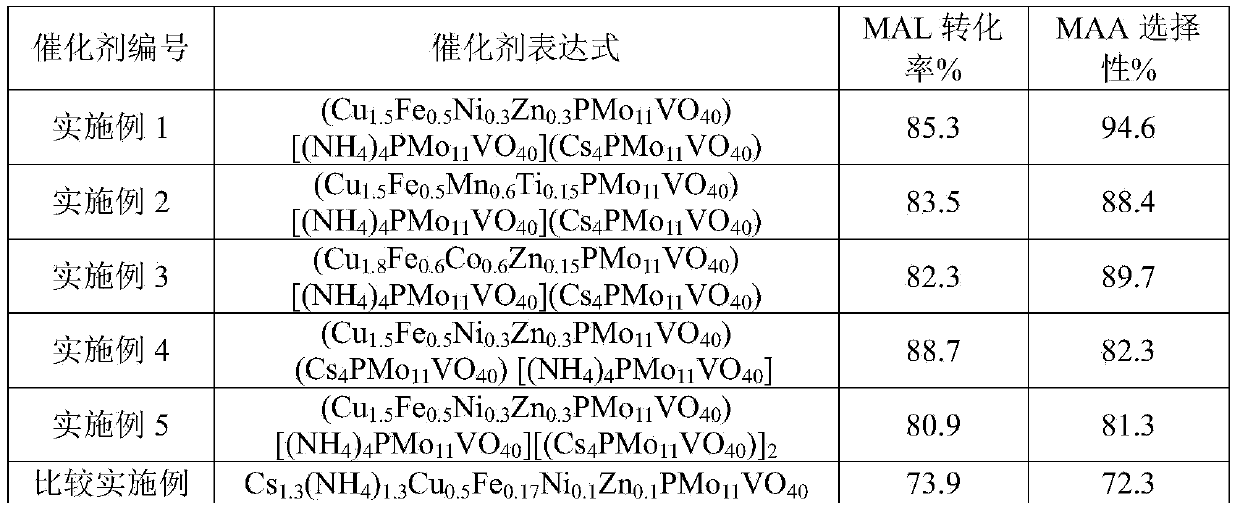
[0036] (Cu 1.5 Fe 0.5 mn 0.6Ti 0.15 PMo 11 VO 40 )[(NH 4 ) 4 PMo 11 VO 40 ](Cs 4 PMo 11 VO 40 )
[0037] Catalyst post-treatment and evaluation methods are as in Example 1. The selectivity was 83.5% for MAL and 88.4% for MAA.
Embodiment 3
[0039] The preparation method of Solution A, Precipitation A and Precipitation B is as in Example 1. Precipitation B is added to the remaining 1 / 3 Solution A, and the temperature is raised to 85°C while stirring, and 59.4g Cu (NO 3 ) 2 , 25.5gFe(NO 3 ) 3 , 23.1gCo(NO 3 ) 2 and 6.0gZn(NO 3 ) 2 Add 1.2 L of deionized water to make a solution, slowly drop it into the suspension, react for 2 hours after the dropwise addition, concentrate and dry, and finally obtain the catalyst precursor B. Its composition expression is:
[0040] (Cu 1.8 Fe 0.6 co 0.6 Zn 0.15 PMo 11 VO 40 )[(NH 4 ) 4 PMo 11 VO 40 ](Cs 4 PMo 11 VO 40 )
[0041] Catalyst post-treatment and evaluation methods are as in Example 1. The selectivity was 82.3% for MAL and 89.7% for MAA.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com