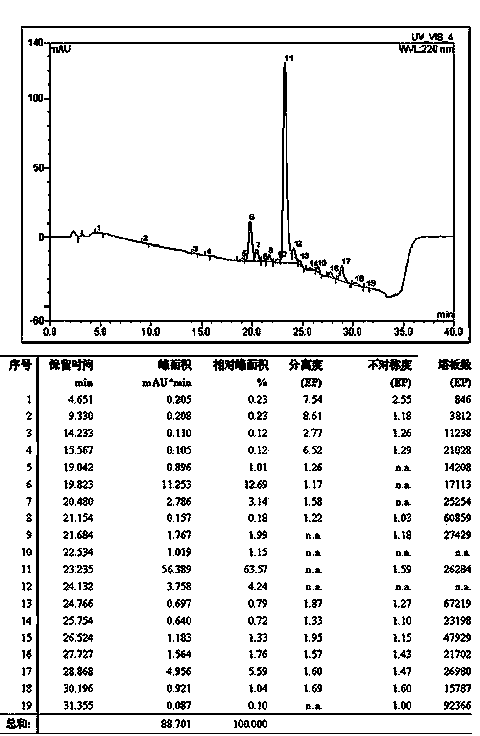
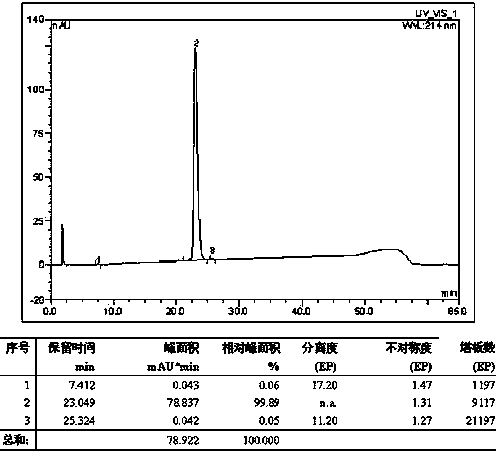
Method for preparing exenatide
A technology of exenatide and preparation process, applied in the field of preparation of polypeptide drugs, can solve the problems of unsuitability for large-scale production, low yield and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099] Example 1: Synthesis of Fmoc-Ser(tBu)-Rink amide AM resin with a degree of substitution of 0.20 mmol / g
[0100] Weigh 5.00 g of Rink amide AM resin (1 mmol) with a substitution degree of 0.20 mmol / g, add it to the solid phase reaction column, wash with DMF once, swell the resin with DCM for 30 minutes, and use a volume ratio of 1:4 The deprotection solution composed of piperidine and DMF was reacted for 5 minutes, DMF was washed once, and the deprotection solution composed of piperidine and DMF in a volume ratio of 1:4 was used to react for 10 minutes. DMF was washed 6 times, and 1.92 g Fmoc was weighed. -Ser(tBu)-OH (5 mmol) and 0.68 g HOBt (5 mmol) were added to a 1:1 volume ratio of DCM and DMF mixed solution, 0.8 ml DIC (5 mmol) was added under ice-water bath for activation and then added to the above In the reaction column of the resin, react for 2 hours, wash 3 times with DMF, 3 times with DCM, 3 times with MeOH, 3 times with DCM, 3 times with MeOH, and dry to ob...
Embodiment 2
[0101] Example 2: Synthesis of Fmoc-Ser(tBu)-Rink amide MBHA resin with a degree of substitution of 0.20 mmol / g
[0102] Weigh 5.00 g of Rink amide MBHA resin (1 mmol) with a substitution degree of 0.20 mmol / g, add it to the solid phase reaction column, wash with DMF once, swell the resin with DCM for 30 minutes, and use a volume ratio of 1:4 The deprotection solution composed of piperidine and DMF was reacted for 5 minutes, DMF was washed once, and the deprotection solution composed of piperidine and DMF in a volume ratio of 1:4 was used to react for 10 minutes. DMF was washed 6 times, and 1.92 g Fmoc was weighed. -Ser(tBu)-OH (5 mmol) and 0.68 g HOBt (5 mmol) were added to a 1:1 volume ratio of DCM and DMF mixed solution, 0.8 ml DIC (5 mmol) was added under ice-water bath for activation and then added to the above In the reaction column of the resin, react for 2 hours, wash 3 times with DMF, 3 times with DCM, 3 times with MeOH, 3 times with DCM, 3 times with MeOH, and dry to ob...
Embodiment 3
[0103] Example 3: Synthesis of Fmoc-Ser(tBu)-Sieber resin with a degree of substitution of 0.20 mmol / g
[0104] Weigh 5.00 g of Rink amide Sieber resin (1 mmol) with a degree of substitution of 0.20 mmol / g, add to the solid phase reaction column, wash with DMF once, swell the resin with DCM for 30 minutes, and use a volume ratio of 1:4 The deprotection solution composed of piperidine and DMF was reacted for 5 minutes, DMF was washed once, and the deprotection solution composed of piperidine and DMF in a volume ratio of 1:4 was used to react for 10 minutes. DMF was washed 6 times, and 1.92 g Fmoc was weighed. -Ser(tBu)-OH (5 mmol) and 0.68 g HOBt (5 mmol) were added to a 1:1 volume ratio of DCM and DMF mixed solution, 0.8 ml DIC (5 mmol) was added under ice-water bath for activation and then added to the above In the resin reaction column, react for 2 hours, wash 3 times with DMF, 3 times with DCM, 3 times with MeOH, 3 times with DCM, 3 times with MeOH, and dry to obtain 5.15 g of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com