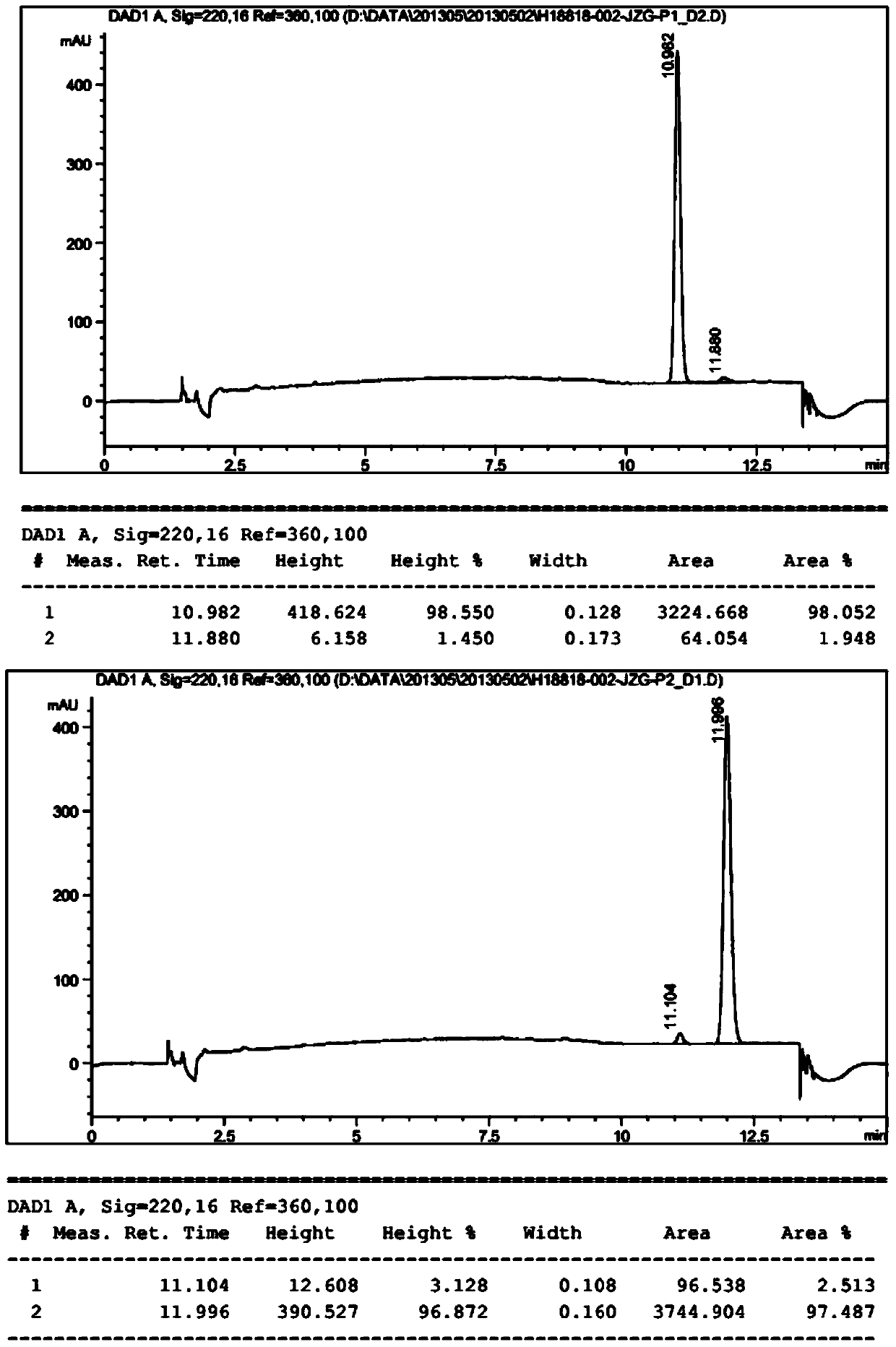
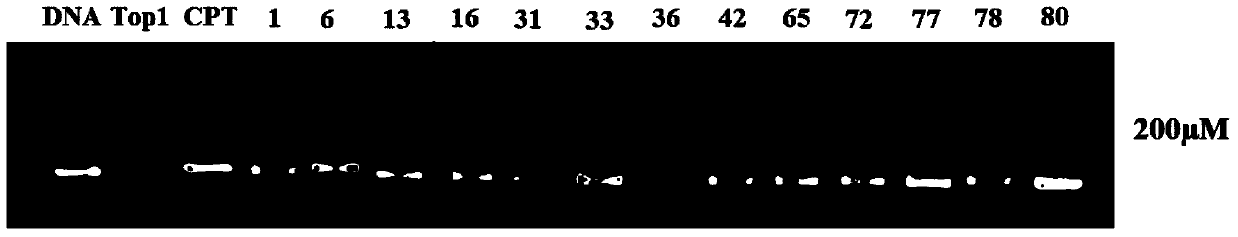
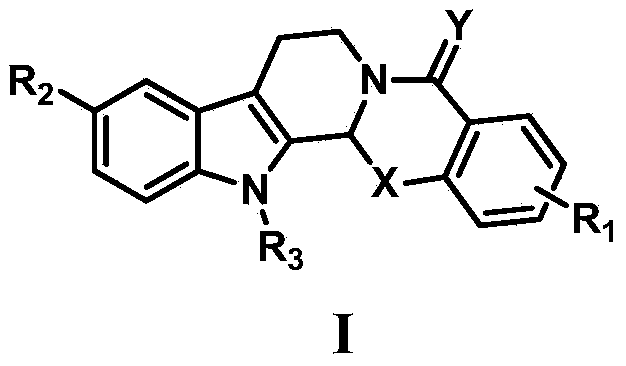
Oxa- or thio-evodiamine anti-tumor derivatives and preparation method thereof
A technology of heteroevodial and derivatives, which is applied in the field of oxa or thiaevodipine antitumor derivatives and their preparation, and can solve the problems of poor water solubility, high toxicity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] Embodiment 1: the synthesis of 15-oxa evodiamine
[0074] A. Preparation of N-formyltryptamine
[0075] In a 50mL three-necked bottle, add 8g (50mmol) tryptamine and 25g ethyl formate, and reflux at 80°C for 12 hours. After the reaction, evaporate the solvent to obtain a brown oily substance, which is left at room temperature for 2-3 days, and crystals slowly appear. Suction filtration obtained 7.3 g of the product with a yield of 87.1%.
[0076] B. Preparation of 3,4-dihydro-β-carboline
[0077] In a 100mL three-necked flask, add 50mL of dichloromethane, add 5g (26mmol) of N-formyltryptamine under stirring conditions, cool to about 5°C in an ice-water bath, then slowly add 12.5mL of phosphorus oxychloride, ice React in the bath for 2 hours, and then react at room temperature for 2 hours. After the reaction, dichloromethane and unreacted phosphorus oxychloride were recovered by distillation under reduced pressure, and the residual solid was extracted three times with ...
Embodiment 2
[0082] Example 2: Synthesis of 13-(4-chlorobenzoyl chloride)-15-oxaevodiamine
[0083] Take 0.30g (1.0mmol) of 15-oxaevodiamine, dissolve it in 15mL of DMF, add 0.04g (1.7mmol) of NaH, stir in ice bath for 10min, add 0.35g (2.0mmol) of p-chlorobenzoyl chloride, and react for 1h Afterwards, the reaction was processed, purified by column chromatography, and the eluent was petroleum ether: ethyl acetate=5:1 to obtain 0.30 g of 13-(4-chlorobenzoyl chloride)-15-oxaevodiamine with a yield of 69.3 %.
Embodiment 3
[0084] Example 3: Synthesis of 10-chloro-15-oxaevodiamine
[0085] Referring to Example 1, the starting material is 5-chlorotryptamine. In step D, 3,4-dihydro-6-chloro-β-carboline and o-hydroxybenzoyl chloride were reacted in dichloromethane at room temperature for 5 h, 0.72 g of 10-chloro-15-oxaevodiamine was obtained with a total yield of 46.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com