Improved methods and related compositions for the synthesis of conjugated polymers by oxidative polymerization
A technology of conjugated polymers and compositions, applied in ultrasonic/acoustic/infrasonic diagnosis, diagnostics, surgical system user interface, etc., can solve the problem of difficult to produce a large amount of processable pure substances, increase costs, development, manufacturing and commercial Complicated process and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
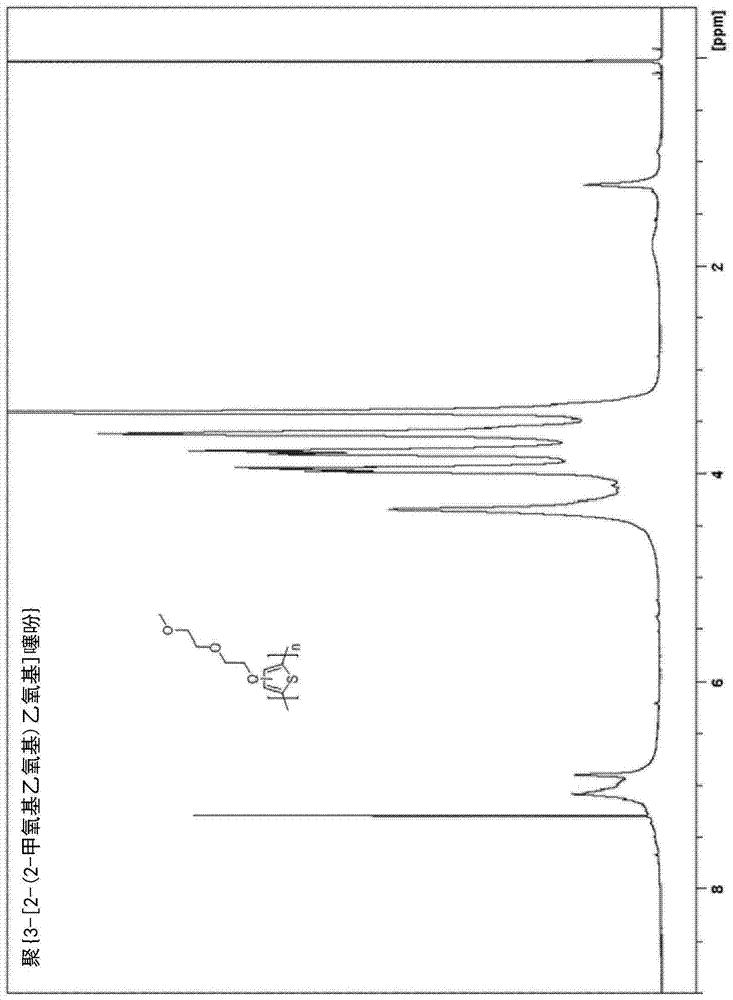
[0185] Example 1. Synthesis of poly{3-[2-(2-methoxyethoxy)ethoxy]thiophene}[PMEET]
[0186]
[0187] A dry 100-mL three-neck flask equipped with an addition funnel was filled with N 2 Rinse and charge 3-[2-(2-methoxyethoxy)ethoxy]thiophene (0.20 g, 1.0 mmol), boron trifluoride etherate (BF 3 ·O(Et) 2 ) (0.5mL, 4.0mmol) and anhydrous CHCl 3 (20 mL, 0.05M). The reaction flask was cooled to 0°C, and 0.05M 2,3-dichloro-5,6-dicyanobenzoquinone (DDQ) in CHCl was added dropwise via a deoxygenated syringe 3 (80 mL, 4.0 mmol) suspension. Formation of a black precipitate was observed within 1 hour of addition of DDQ. After the addition was complete, stirring was continued for 24 hours, at which point the reaction mixture was poured into 200 mL of hexane, forming fine black particles. The combined organic solids were washed twice with hexane and dried. The crude product was redissolved in a minimum amount of methanol and precipitated in water. The polymer was collected by filt...
Embodiment 2
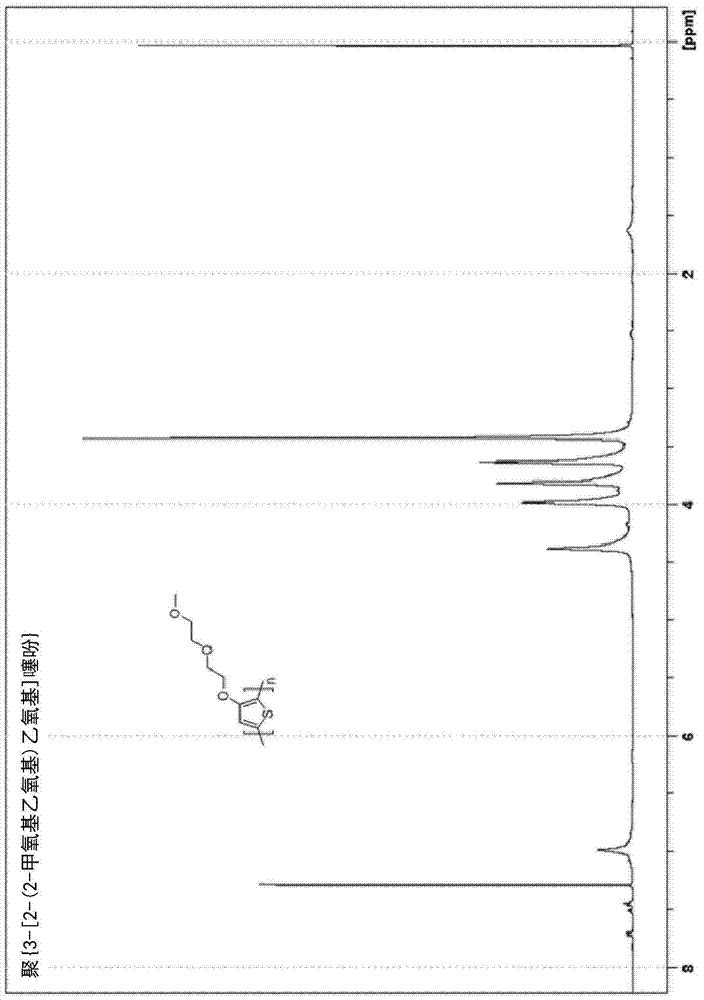
[0190] Example 2. Solvent-free synthesis of poly{3-[2-(2-methoxyethoxy)ethoxy]thiophene}[PMEET]
[0191]
[0192] A dry 250-mL three-neck flask equipped with a mechanical stirrer and a thermocouple was filled with N 2 Rinse and charge 3-[2-(2-methoxyethoxy)ethoxy]thiophene (20 g, 0.099 mol) and boron trifluoride etherate (BF 3 ·O(Et) 2 ) (50 mL, 0.41 mol). 2,3-Dichloro-5,6-dicyanobenzoquinone (DDQ) (27.86 g, 0.123 mol) was added in five portions. The reaction was exothermic and DDQ was added in such a way that the reaction temperature was allowed to stabilize and / or begin to decrease before each successive addition. The formation of a black precipitate was observed when the first portion of DDQ was added. After the addition was complete, the temperature was set at 60°C and stirring was continued for 24 hours, at which point most of the material solidified. The reaction mixture was quenched by adding zinc dust (16.1 g, 0.246), and the reaction mixture was stirred for an a...
Embodiment 3
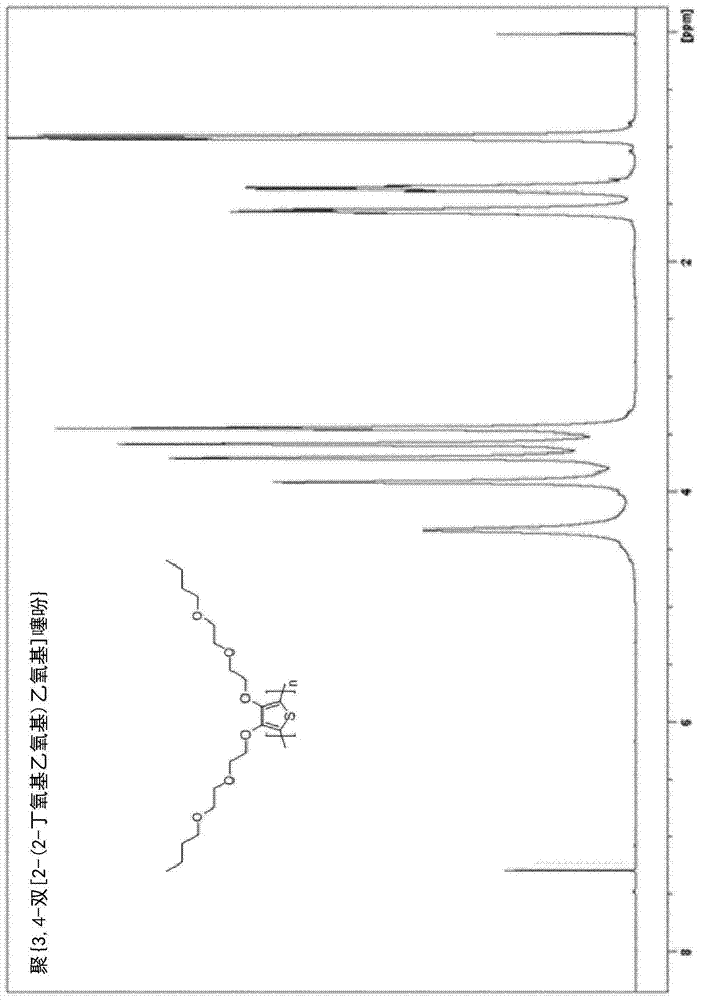
[0194] Example 3. Synthesis of poly{3,4-bis[2-(2-butoxyethoxy)ethoxy]thiophene}[PdiBEET]
[0195]
[0196] Fill a dry 100-mL three-neck flask with N 2 Rinse, and charge 3,4-bis[2-(2-butoxyethoxy)ethoxy]thiophene (1.83 g, 4.50 mmol), boron trifluoride etherate (BF 3 ·O(Et) 2 ) (1.40mL, 11.1mmol) and anhydrous CHCl 3 (20 mL, 0.23M). 2,3-Dichloro-5,6-dicyanobenzoquinone (DDQ) (3.1 g, 13.6 mol) was added in small portions. Polymerization was performed at room temperature (approximately 25°C). The formation of a black precipitate was observed within 1 hour of adding DDQ. After the addition was complete, stirring was continued for 24 hours and the reaction mixture was poured into 200 mL of a water:methanol (50:50) mixture to quench and precipitate the polymer. The organic solid was separated and washed well with hot water. The residue was dedoped by stirring in 100 mL of water containing 2 mL of hydrazine monohydrate for 1 hour. Redissolve the polymer in a minimal amount ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com