A method for converting cholesterol into cholest-4-en-3-one by using whole cells of recombinant bacillus subtilis
A cholesterol and recombinant vector technology, applied in the fields of genetic engineering and enzyme engineering, can solve problems such as potential safety hazards, and achieve the effects of easy control, high utilization rate of raw materials, and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1: Construction of recombinant B.subtilis168 bacterial strain
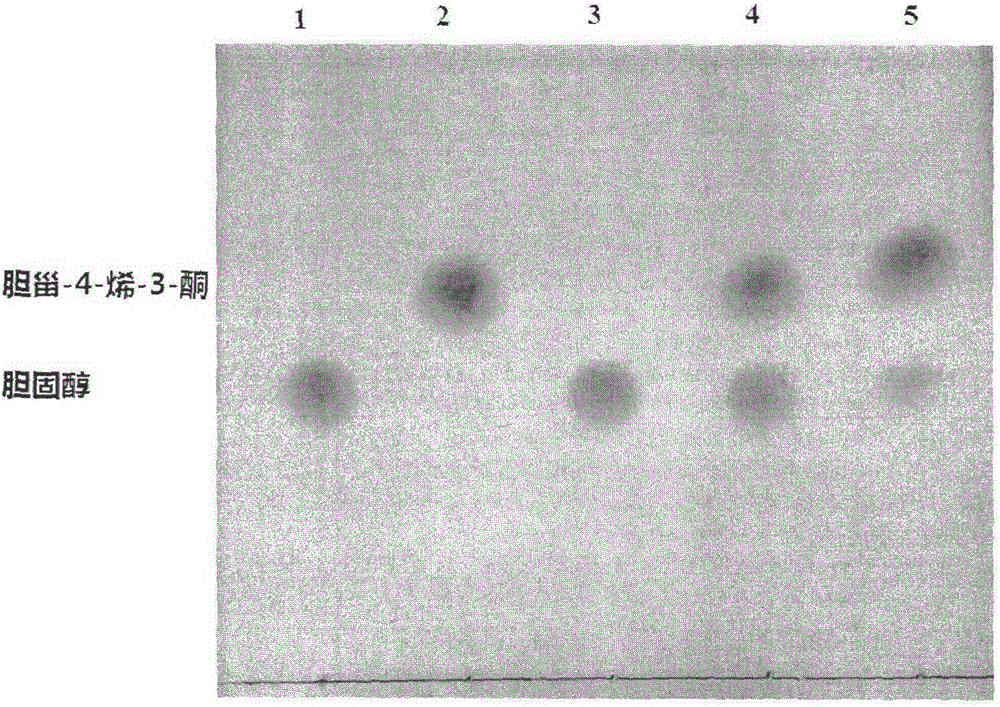
[0039] The new Mycobacterium aureus with cholesterol oxidase activity in the laboratory was used as the starting strain, and its chromosome was used as a template to obtain the gene of the enzyme by means of PCR, and then connected to the cloning vector to achieve a large amount of amplification of the gene. A large number of amplified KSDD gene fragments were purified and connected to the pMA5 vector. After successful verification, the model strain Bacillus subtilis 168 was transformed. Positive transformants were screened on the resistance plate, inoculated in shake flasks for fermentation, and the product cholest-4-en-3-one in the fermentation broth was detected by TLC and HPLC. The product cholest-4-en-3-one was detected, indicating that the recombinant strain capable of converting cholesterol into cholest-4-en-3-one was successfully constructed. The present invention finally obtains the B. ...
Embodiment 2
[0040] Embodiment 2: the enzyme activity assay of recombinant bacterial strain
[0041] The strain was cultured overnight in LB medium, centrifuged at 8000 rpm for 10 min, washed 3 times with 50 mM Tris-HCl buffer solution of pH 7.0, and resuspended in 5 ml of the buffer solution. Ultrasonic crushing with 40% power in an ice bath, working for 2 seconds and resting for 5 seconds, and working time for 10 minutes. Centrifuge at 10,000r / min for 30min to obtain the supernatant, which contains the target protein. The enzyme activity determination method is as follows: 3mL solution A (4-amino-antipyrine 1mmol / L; phenol 6mmol / L; sodium azide 0.2g / L; peroxidase 5000U / L; potassium phosphate buffer 25mmol / L , pH 7.5), 150 μL solution B (cholesterol 8.26g / L; Triton X-100 4.26%; isopropanol as solvent), 50 μL enzyme solution, reacted at 37°C for 5min, boiled water bath for 3min, and measured the absorbance value OD at 500nm 500 . Definition of enzyme activity unit: at 37°C, the amount o...
Embodiment 3
[0042] Example 3: Detection of whole cell transformation performance of recombinant strains
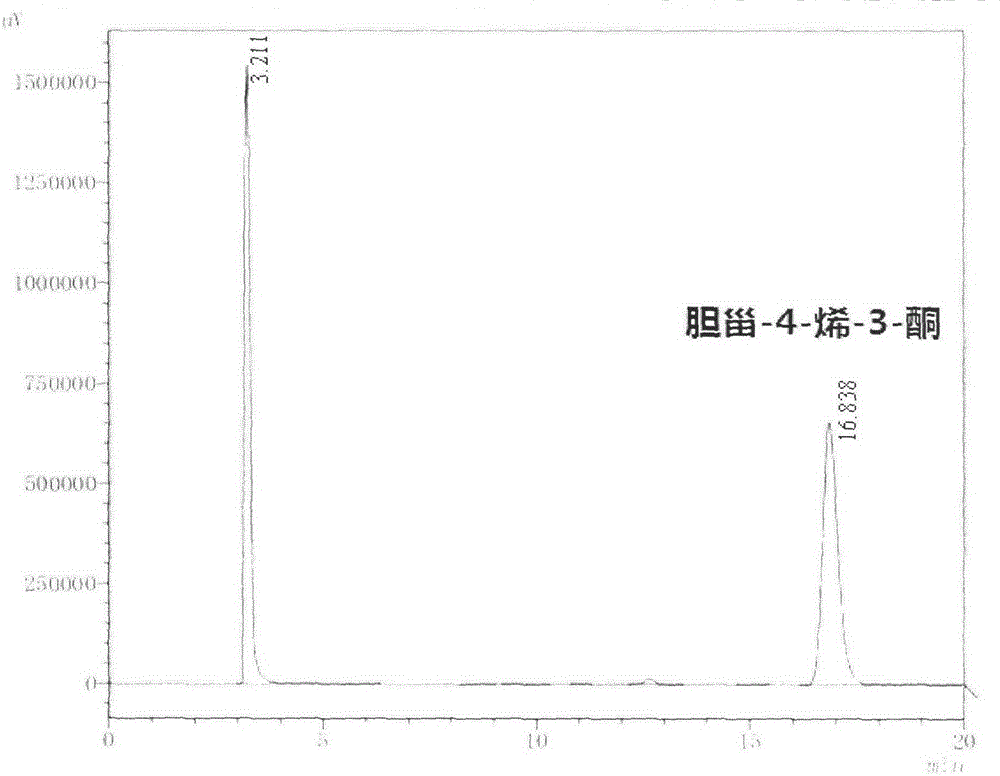
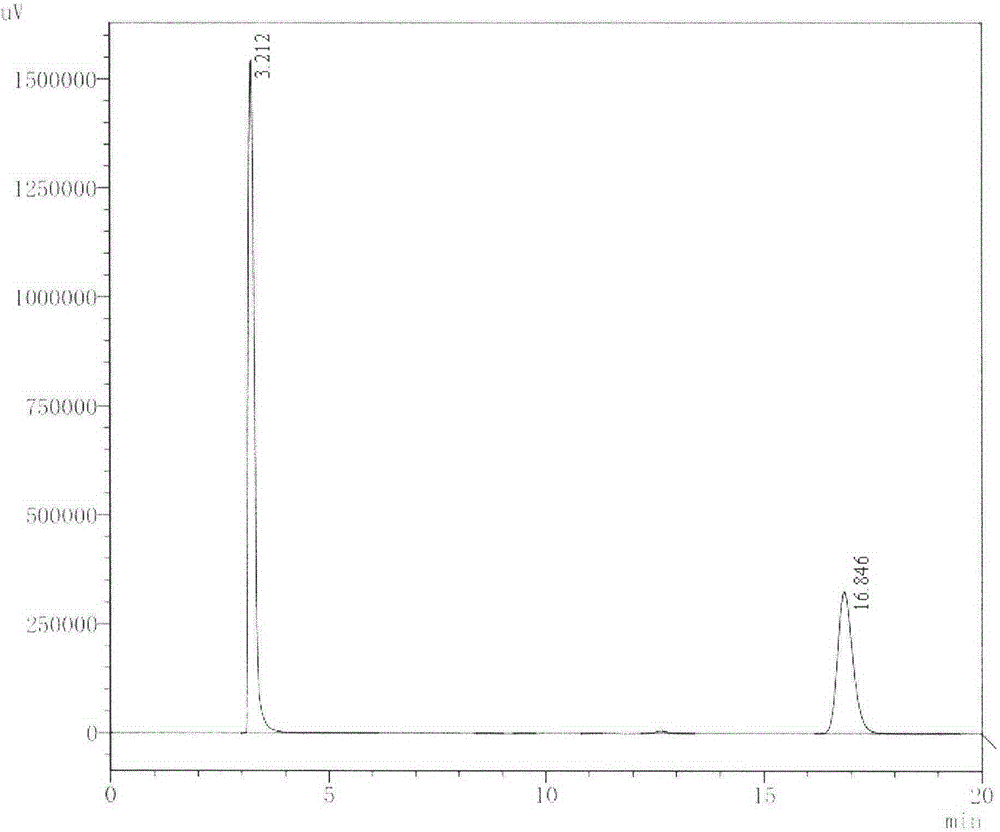
[0043] The bacterial strain was inoculated in 100mL LB medium with 1% inoculum, cultivated for 24h, recovered the thalli by centrifugation, washed twice, resuspended with an appropriate 50mM Tris-HCl (pH7.0) buffer, added 0.1% ( w / v) cholesterol and 0.1% (v / v) Tween-80, continue to culture at 37°C, 160rpm for 24h. After detection and analysis by HPLC, such as image 3 As shown, the final substrate molar conversion rates reached 67% and 83%, respectively, which were about 20 and 25 times higher than that of the original bacteria.
[0044]
[0045]
[0046]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com