Method for improving thermal stability of bacillus subtilis lipase A
A Bacillus subtilis, thermal stability technology, applied in the field of enzyme engineering, can solve the problems of complex process, limit the work progress in the field of protein transformation, large screening capacity, etc., achieve improved thermal stability, avoid large screening capacity, and simplify rational design The effect of the method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
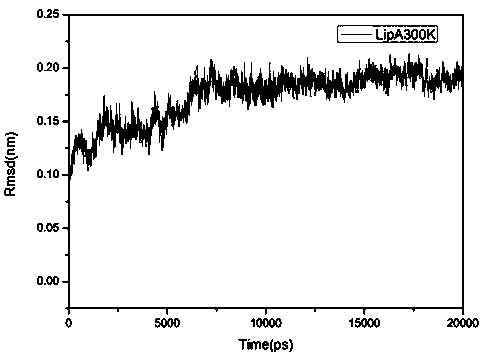
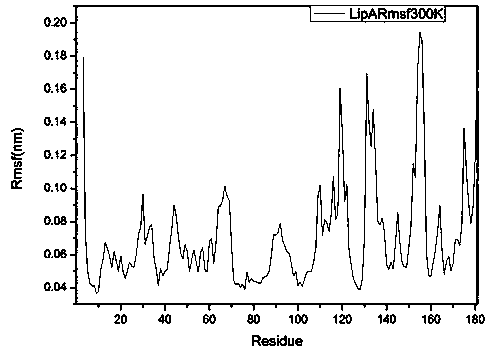
[0024] This example illustrates the method of obtaining the crystal structure of Bacillus subtilis lipase A in step 1) of the present invention and analyzing the loop region with higher flexibility in it by means of molecular dynamics. Bacillus subtilis lipase A ( Bacillus subtilis LipA, PDB: 1I6W) was the research target, and the crystal structure of Bacillus subtilis lipase A was obtained by searching the RCSB database; the obtained Bacillus subtilis lipase A was subjected to 20 ns molecular dynamics simulation ( figure 2 ), and extract the equilibrium time period to analyze the root mean square fluctuation RMSF of Bacillus subtilis lipase A ( image 3 ).
Embodiment 2
[0026] This example illustrates the method of determining the glycine (Gly) residue in the highly flexible Loop region as the mutation site by proline-binding effect analysis in step 2) of the present invention. According to the RMSF diagram of Bacillus subtilis lipase A obtained in step 1), the Pymol visualization software combined with the "proline effect" theory analysis, finally, the results of the mutation sites obtained in this round of screening are: Gly153, Gly155, Gly158, Gly111, Gly116, Gly46, Gly52.
Embodiment 3
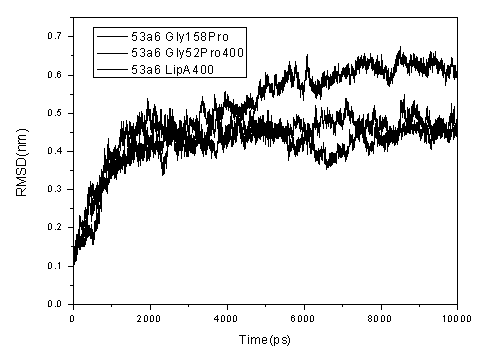
[0028] This example illustrates the method of step 3) through the molecular dynamics simulation analysis, the method of screening the disabling mutation of Gly to Pro on the thermostability of Bacillus subtilis lipase A.
[0029] The Gly153Pro, Gly155Pro, Gly158Pro, Gly111Pro, Gly116Pro, Gly46Pro, and Gly52Pro mutants were constructed using the online server SWISS-Model, and the constructed mutant models were evaluated and optimized using Verify_3D. The kinetic simulation of Bacillus subtilis lipase A and its mutants was carried out using the GROMACS4.5.4 software package. The simulation steps mainly include the following steps:
[0030] The first step uses the pdb2gmx command to add missing hydrogen atoms in the protein. And use the cube box to fill the SPC solvent water model and the GROMOS9653a6 force field. The command is:
[0031] pdb2gmx -f LipA.pdb -o.gro -p LipA.top -i .itp -water spc -ignh
[0032] editconf -bt cubic -f LipA.gro -o LipA.gro -d 0.9
[0033] The se...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com