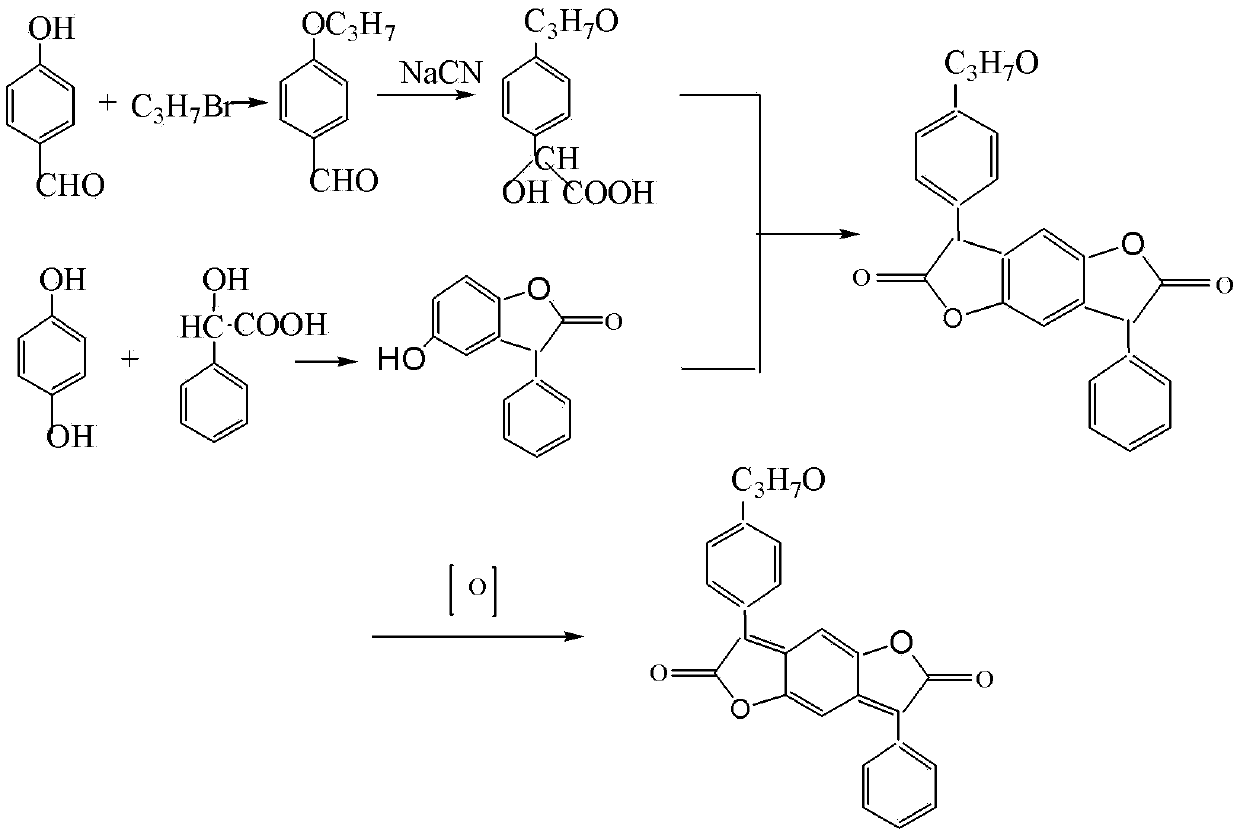
Disperse red 356 preparation method
A technology of disperse red and mass ratio, which is applied in the direction of chemical instruments and methods, organic dyes, azo dyes, etc., can solve the problems of low yield and high production cost, achieve purity and yield improvement, reduce the production of by-products, The effect of increasing productivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] The synthesis of step 1 p-propoxybenzaldehyde:
[0042] In a 1000ml four-neck bottle with mechanical stirring and a thermometer, add toluene 250, DMF200 mixed solvent, start stirring, and then add 50g of p-hydroxybenzaldehyde, 50g of bromopropane, 2g of auxiliary chlorine-type strong alkali ion exchange resin, hydrogen Potassium oxide 20g, the temperature was raised to 85°C for 1 hour, and after the reaction was completed, the temperature was lowered to 10°C, water was added, stirred for 30 minutes, and allowed to stand for stratification. The upper toluene layer was washed twice with water to obtain 66.5 g of p-propoxybenzaldehyde with a content of 99% and a yield of 98%.
Embodiment 2
[0044] The synthesis of step 1 p-propoxybenzaldehyde:
[0045] In a 1000ml four-neck bottle with mechanical stirring and a thermometer, add toluene 400, DMF300 mixed solvent, start stirring, and then add 50g of p-hydroxybenzaldehyde, 60g of bromopropane, 4g of auxiliary agent chlorine-type strong alkali ion exchange resin, hydrogen Potassium oxide 25g, the temperature was raised to 95°C for 1 hour reaction, after the reaction was completed, the temperature was lowered to 15°C, water was added, stirred for 30 minutes, and allowed to stand for stratification. The upper toluene layer was washed twice with water, and 66.8 g of p-propoxybenzaldehyde was obtained by precipitation, with a content of 98.5% and a yield of 98%.
Embodiment 3
[0047] The synthesis of step 1 p-propoxybenzaldehyde:
[0048]In a 1000ml four-necked bottle with mechanical stirring and a thermometer, add toluene 500, DMF250 mixed solvent, start stirring, and then add 50g of p-hydroxybenzaldehyde, 65g of bromopropane, 3g of auxiliary chlorine-type strong alkali ion exchange resin, hydrogen Potassium oxide 30g, the temperature was raised to 110°C to react for 1 hour, and after the reaction was completed, the temperature was lowered to 25°C, water was added, stirred for 30 minutes, and allowed to stand for stratification. The upper toluene layer was washed twice with water to obtain 66.8 g of p-propoxybenzaldehyde with a purity of 97.5% and a yield of 97%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com