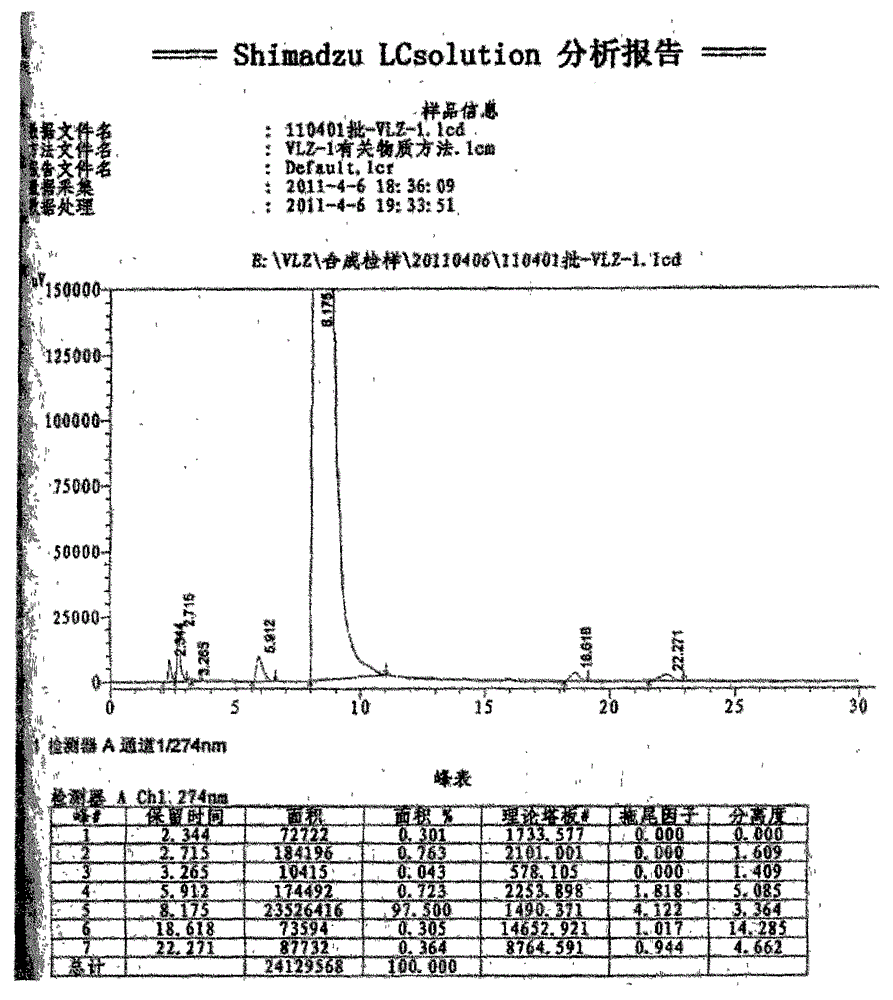
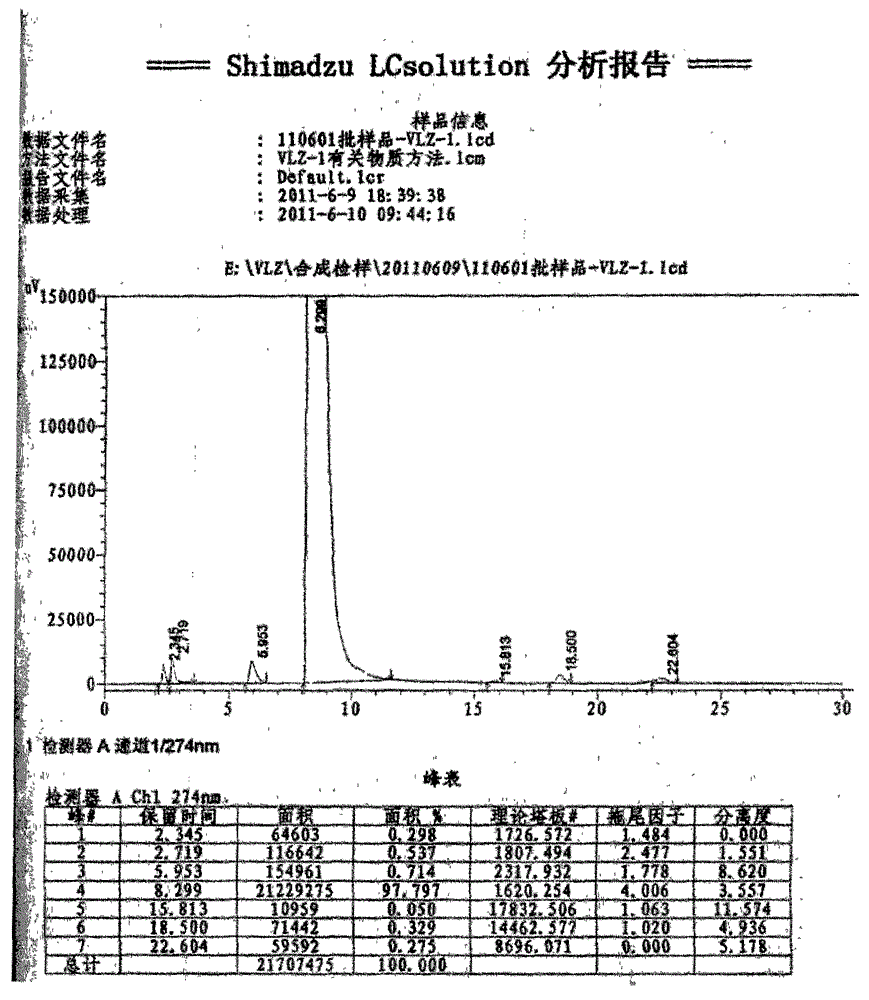
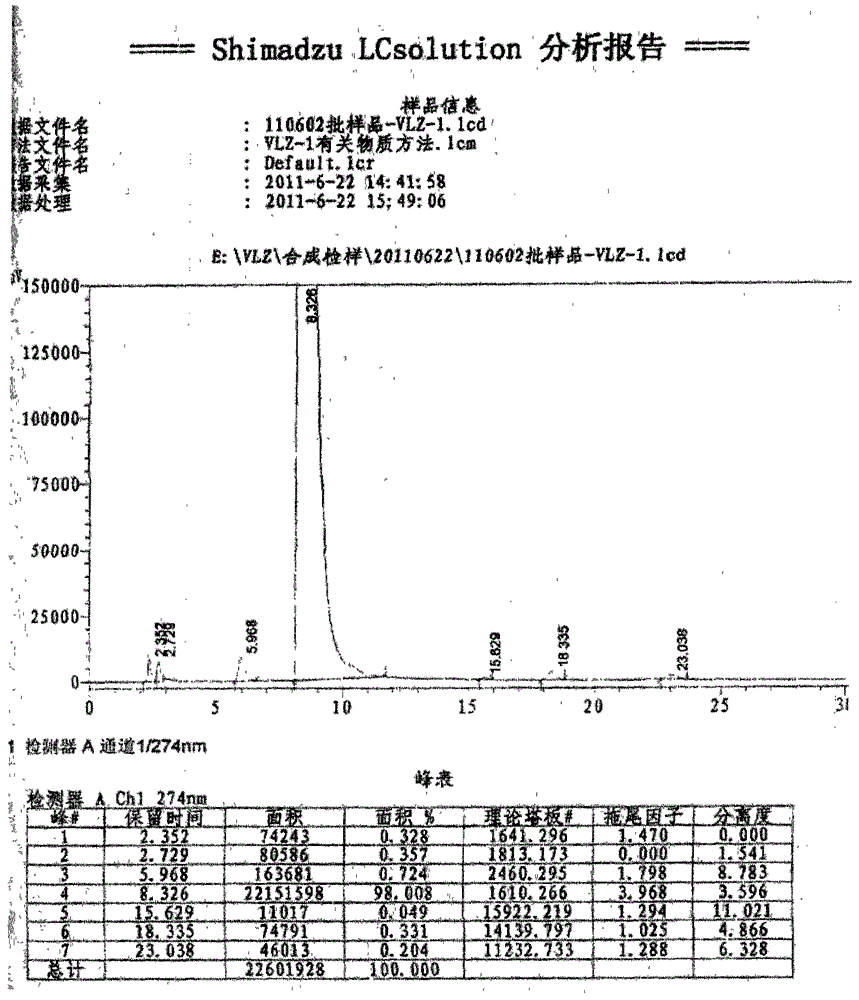
A kind of synthetic method of ethyl 5-(piperazin-1-yl)benzofuran-2-carboxylate
A technology of benzofuran and ethyl carboxylate, which is applied in the field of synthesis of pharmaceutical intermediates, can solve the problems of high production cost, low product purity, low yield and the like, achieves simple reaction control conditions and post-processing process, and is concise. Operation, Effects of Raw Materials in Broad Synthetic Routes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1: Preparation of 3-[2-hydroxyl-5-(piperazin-1-yl)]phenyl-2-propenal (1)
[0034] N 2 Under air protection, add 40g (0.6mol) zinc and 2LTHF to a dry four-neck flask with magnetic stirring, start stirring, cool the reaction system to -20~-15°C, and drop 32.5mL (0.3mol) TiCl 4 , Control the temperature of the system at -20~-15°C during the dropping process. After the dropwise addition, the temperature was raised to reflux for 3h, and again under N 2 Cool the system down to -20~10°C under protection, add dropwise 75ml of a THF solution containing 24.8g (0.12mol) of 2-hydroxy-5-(piperazin-1-yl)benzaldehyde and 7g (0.12mol) of glyoxal , and heated to reflux for 2 hours after dropping, followed by TLC until the end of the reaction.
[0035] The reaction solution was filtered to remove solids, after concentration, 500ml of saturated sodium bicarbonate solution was added, stirred with an electric stirrer for half an hour, 400ml of chloroform was added to extract 3 ti...
Embodiment 2
[0036] Example 2: Preparation of 3-[2-hydroxyl-5-(piperazin-1-yl)]phenyl-2-propenal (2)
[0037] N 2 Under air protection, add 80g (1.2mol) of zinc and 4LTHF to a dry four-neck flask with magnetic stirring, start stirring, cool the reaction system to -14~-10°C, and drop in 65mL (0.6mol) of TiCl 4 , Control the temperature of the system at -14~-10°C during the dropping process. After the dropwise addition, the temperature was raised and refluxed for 4.5h, again under N 2 Cool the system to -20~10°C under protection, add dropwise 150ml of a THF solution containing 49.6g (0.24mol) of 2-hydroxy-5-(piperazin-1-yl)benzaldehyde and 14g (0.24mol) of glyoxal , and heated to reflux for 5 hours after dropping, followed by TLC until the end of the reaction.
[0038]The reaction solution was filtered to remove solids, after concentration, 1000ml of saturated sodium bicarbonate solution was added, stirred with an electric stirrer for half an hour, 600ml of chloroform was added to extract...
Embodiment 3
[0039] Example 3: Preparation of 3-[2-hydroxyl-5-(piperazin-1-yl)]phenyl-2-acrylic acid (1)
[0040] Add 40ml of 10% sodium hydroxide solution into a 100ml three-neck flask, drop in 4ml of 5% copper sulfate solution, and stir for 20 minutes with an electric stirrer, the reaction system turns blue. Add 39.4 g (0.17 mol) of 3-[2-hydroxyl-5-(piperazin-1-yl)]phenyl-2-propenal dropwise to the reaction system while stirring. After the drop is complete, heat and reflux for 30 minutes, and the solution It turns yellow, and the solution color is brownish red after cooling, with red precipitate. Filter out the red precipitate, neutralize the filtrate with 1mol / L hydrochloric acid until the pH is 6-7, extract 3 times with 50ml of chloroform respectively, combine the organic phases, decolorize with activated carbon under reflux, filter, dry the filtrate with anhydrous sodium sulfate, and concentrate Chloroform was recovered to obtain 45 g of yellow oil, which contained 88% of 3-[2-hydrox...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com