Thieno (2, 3-f) benzofuran compound, polymers thereof and application of polymers
A technology of benzofuran and 3-f, which is applied in the field of organic optoelectronic material synthesis, can solve problems such as few reports, achieve great commercial prospects, good thermal stability, and improve solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
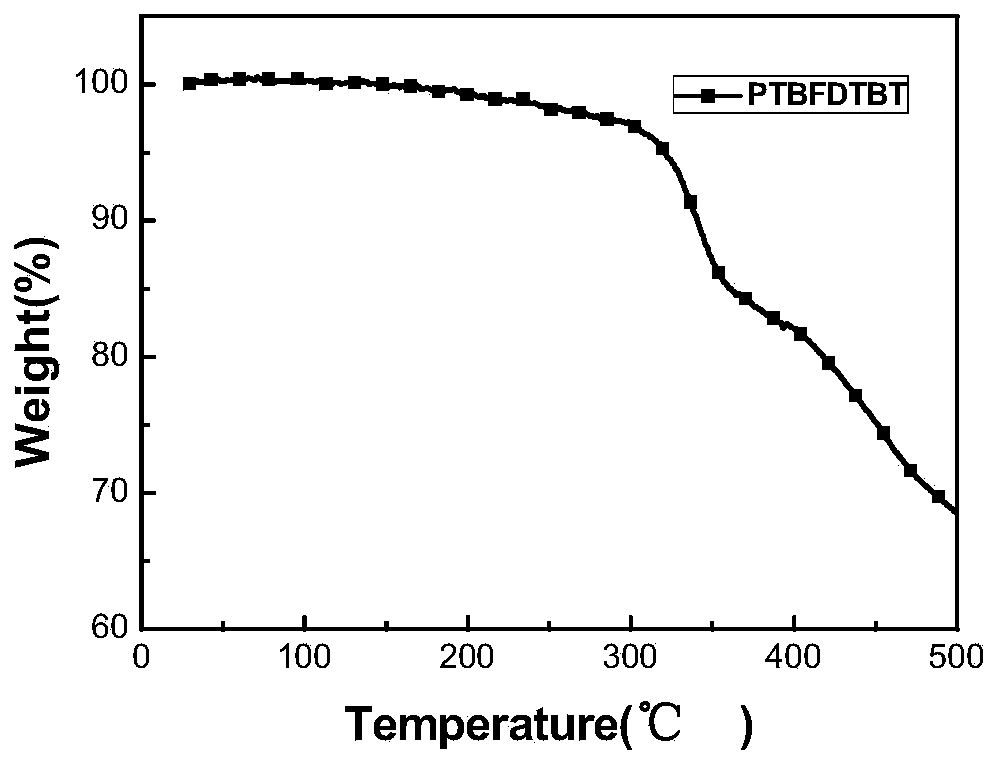
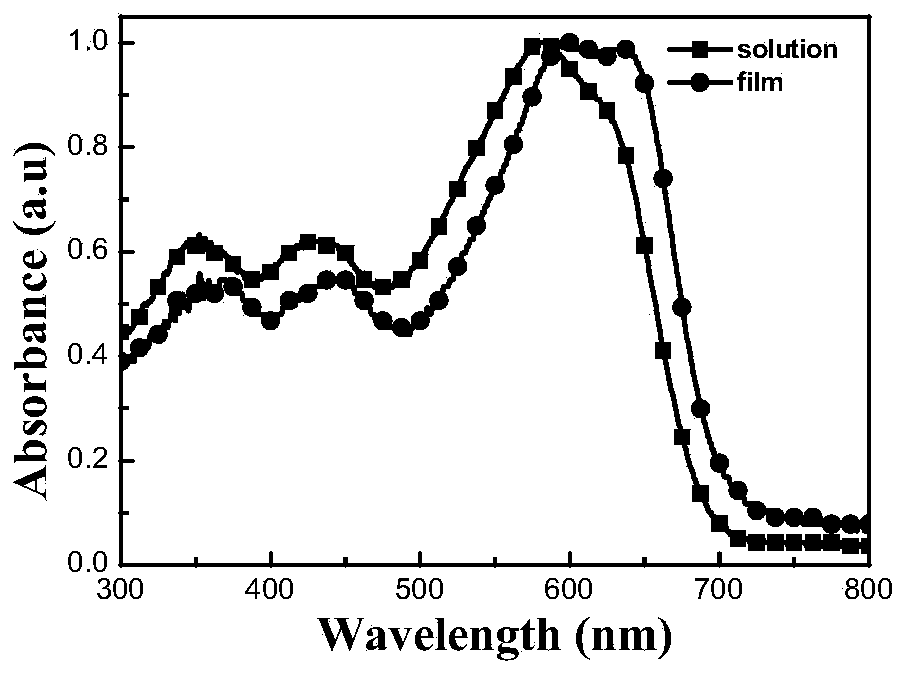
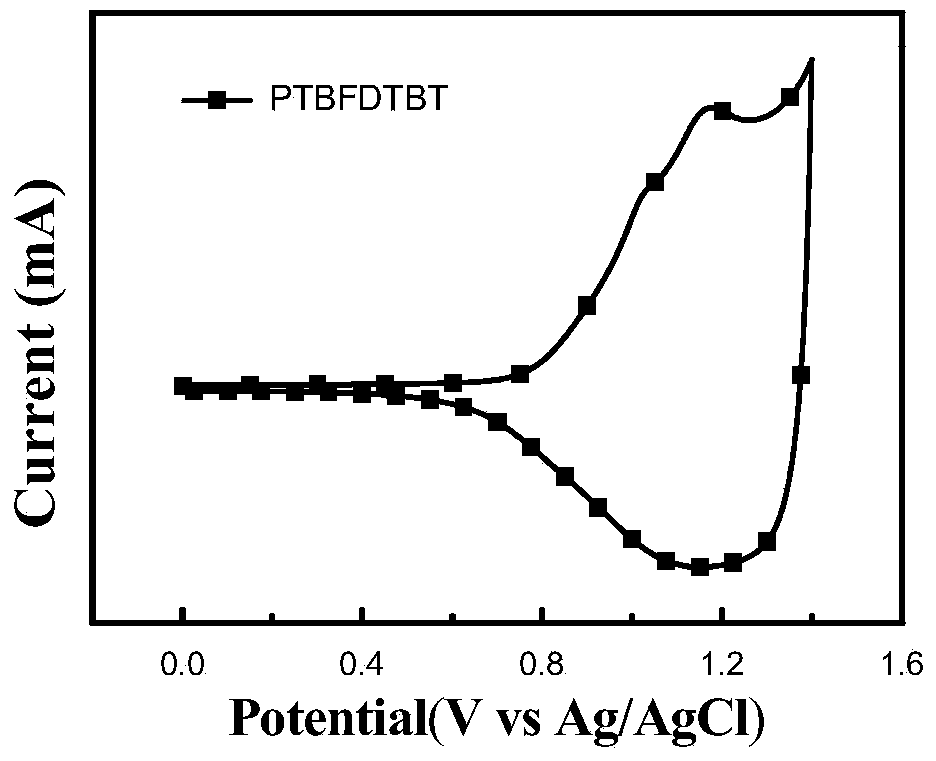
Image
Examples
Embodiment 1
[0051] a) Thionyl chloride (65.3mL, 0.9mol) was added dropwise into a 500ml dry round-bottomed flask of furan-3-carboxylic acid (25.1g, 0.224mol), stirred and refluxed for 4 hours, then cooled to room temperature Finally, the solvent was spin-dried to obtain compound 1 as a colorless oil, which was used in the next step without further purification.
[0052] b) Compound 1 (29.24g, 224mmol) was added dropwise to a solution of diethylamine (92.6mL) in dichloromethane (100mL) at 0°C, and then the reaction mixture was warmed to room temperature and stirred for 1 hour. After cooling to room temperature, the mixture was poured into ice water, extracted with dichloromethane, the organic phase was washed twice with water, dried over anhydrous magnesium sulfate, and the solvent was spin-dried, and the crude product was passed through a silica gel column (eluted with ethyl acetate / petroleum ether (2:1 , V / V) is the eluent) to obtain compound 2 (7.5 g, 85%) as an orange-red oil. 1 H NMR...
Embodiment 2
[0058] The synthesis method of thieno[2,3-f]benzofuran compound is as in Example 1.
[0059] Polymer (PTBFDTBTz) was prepared by Stille reaction: under nitrogen protection, M1 (0.134g, 0.151mmol) and N-octyl-4,7-dithienyl-benzotriazole dibromide (0.0838g, 0.151g) Add to 10mL of anhydrous toluene and 2ml of DMF, then add Pd(PPh 3 ) 4 (10mg), stirred and reacted at 110°C for 24 hours, cooled to room temperature, poured into 100mL methanol for precipitation, filtered, extracted with methanol, n-hexane, and chloroform sequentially in a Soxhlet extractor, recovered the chloroform solution, and spin-dried Add a small amount of chloroform to dissolve the excess solvent, pour it into a centrifuge tube, add methanol to make it chromatographically, pour off the upper clear night after high-speed centrifugation, and repeat several times to obtain the target polymer PTBFDTBTz (91mg, 62%). m n = 2.8 kDa; M w = 21.88 kDa; PDI = 7.8. Anal. Calcd for (C 58 h 69 N 3 OS 5 )n (%): C, 70...
Embodiment 3
[0061] The synthesis method of thieno[2,3-f]benzofuran polymer is as in Example 1.
[0062] Polymer (PTBFDTBO) was prepared by Stille reaction: under nitrogen protection, M1 (0.1359g, 0.153mmol) and 5,6-dioctyloxy-4,7-dithienyl-benzoxadiazole dibromide (0.1068 g, 0.153mmol) was added to 10mL of anhydrous toluene and 2 ml of DMF, and then Pd (PPh 3 ) 4 (10mg), stirred and reacted at 110°C for 24 hours, cooled to room temperature, poured into 100mL methanol for precipitation, filtered, extracted with methanol, n-hexane, and chloroform sequentially in a Soxhlet extractor, recovered the chloroform solution, and spin-dried Add a small amount of chloroform to dissolve the excess solvent and pour it into a centrifuge tube, add methanol to make it chromatographically come out, pour off the upper layer after high-speed centrifugation, and repeat several times to obtain the target polymer PTBFDTBO (89mg, yield 52%) . m n = 524.1 kDa; M w = 1011.5 kDa; PDI = 1.9. Anal. Calcd for (C...
PUM
| Property | Measurement | Unit |
|---|---|---|
| energy conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com