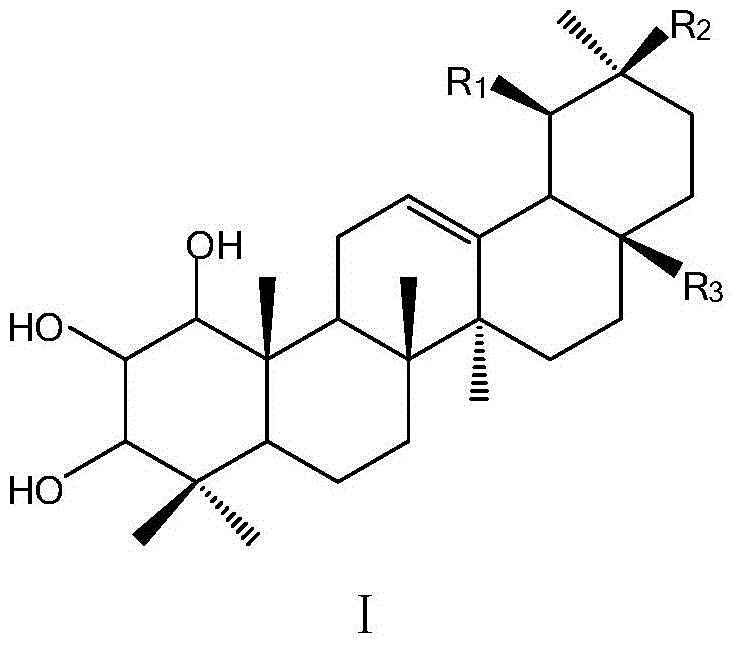
Application of a ring trihydroxyl substituted pentacyclic triterpene compound in pharmacy
A pentacyclic triterpene and trihydroxy technology, which is applied in the field of preparation of antibacterial or anti-tobacco mosaic virus drugs, can solve the problems of anti-plant pathogenic bacteria and anti-tobacco mosaic virus research that have not been reported.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
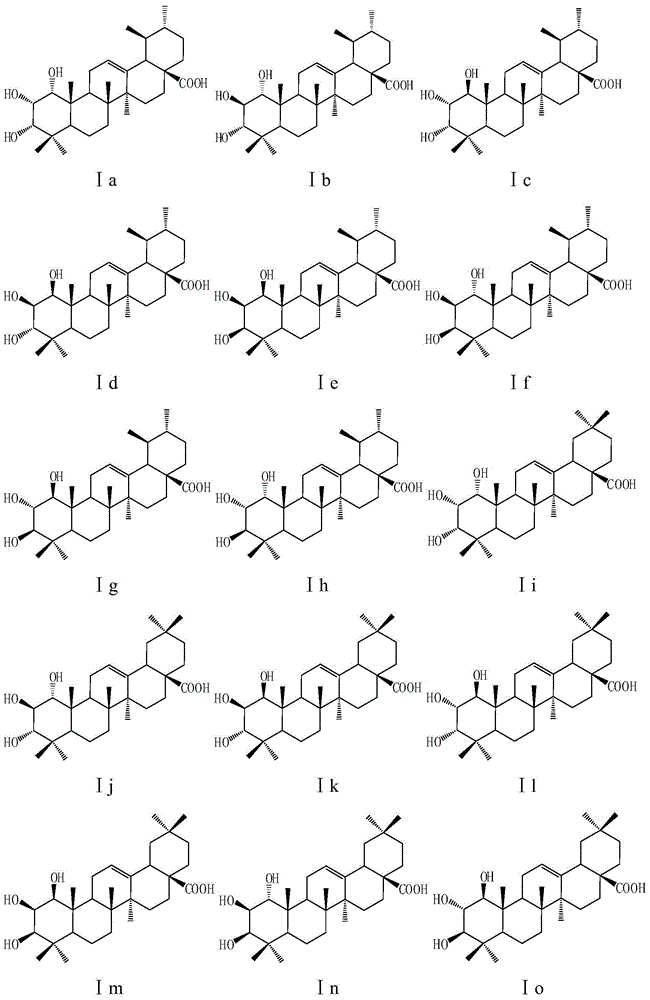
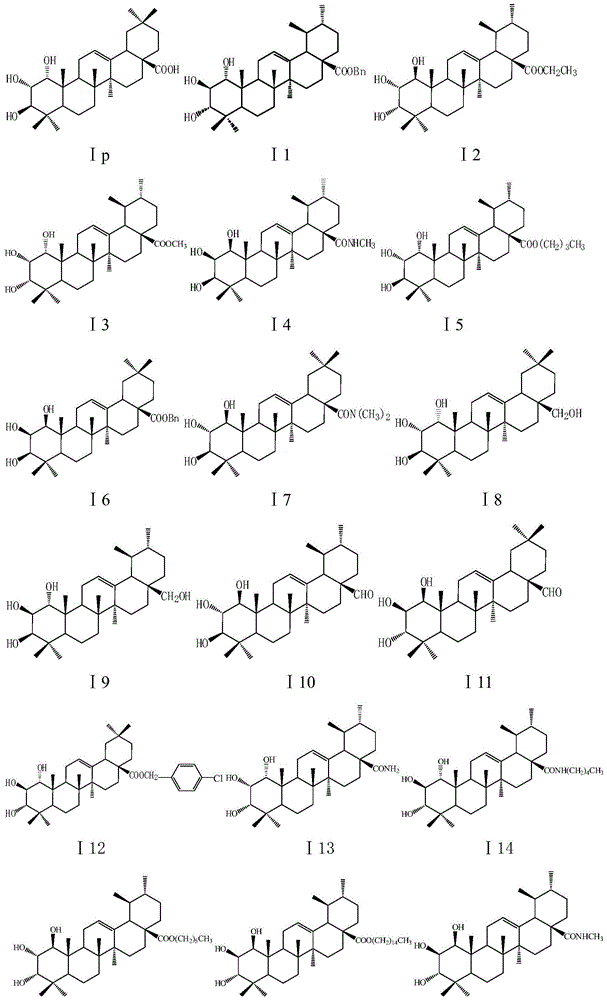
[0161] Embodiment 1 of the present invention: take any one of compound I17, U2, U4, A2, A3, add appropriate amount of starch and microcrystalline cellulose according to the pharmaceutical method and mix well, mix the hydroxymethyl cellulose solution with the above-mentioned Mix the powder, pass through 80-mesh sieve to obtain wet granules, dry at 50-60°C, pre-screen carboxymethyl starch salt, magnesium stearate and talcum powder, then add to the above granules and press into tablets to obtain tablets . The preparation is taken orally, twice a day, and the dose of each dose is 5-30 mg based on the crude drug. For the treatment of infectious diseases caused by Staphylococcus aureus such as suppurative infection, pneumonia, pseudomembranous enteritis, pericarditis, etc.
Embodiment 2
[0162] Example 2: Take any one of the compounds I3, I15, I18, I23, U1, U3, U5, and A4, add an appropriate amount of sodium citrate, polyethylene glycol, and distilled water according to the pharmaceutical method, mix well and fully dissolve, adjust The pH of the solution is 7.5-8.5, filtered, the concentration of the crude drug is 1 mg / ml, divided into 2 ml per ampoule, and sterilized to obtain the injection. The preparation is used for injection, twice a day, and the amount of each injection is 5-30 mg based on the raw drug. For the treatment of infectious diseases caused by Staphylococcus aureus.
Embodiment 3
[0163] Embodiment 3: Take any one of the compounds Ia, Ic, Ij, Io, Ip, I13, A1, A5, A7, pulverize through an 80 mesh sieve, add an appropriate amount of vegetable oil containing 10% beeswax according to the pharmaceutical method, and mix well ; Prepare capsule material with gelatin: glycerin: distilled water: preservative = 1: 0.4: 0.8: 0.003, add the mixture of the aforementioned compounds and vegetable oil, and compress to obtain soft capsules. The preparation is taken orally, twice a day, and the dose of each dose is 5-30 mg based on the crude drug. For the treatment of infectious diseases caused by Staphylococcus aureus.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com