Humanized universal light chain mice
A humanized, mouse technology, applied in the field of humanized universal light chain mice, can solve problems such as unfavorable expression or binding performance of two heavy chains, and no solution.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment I
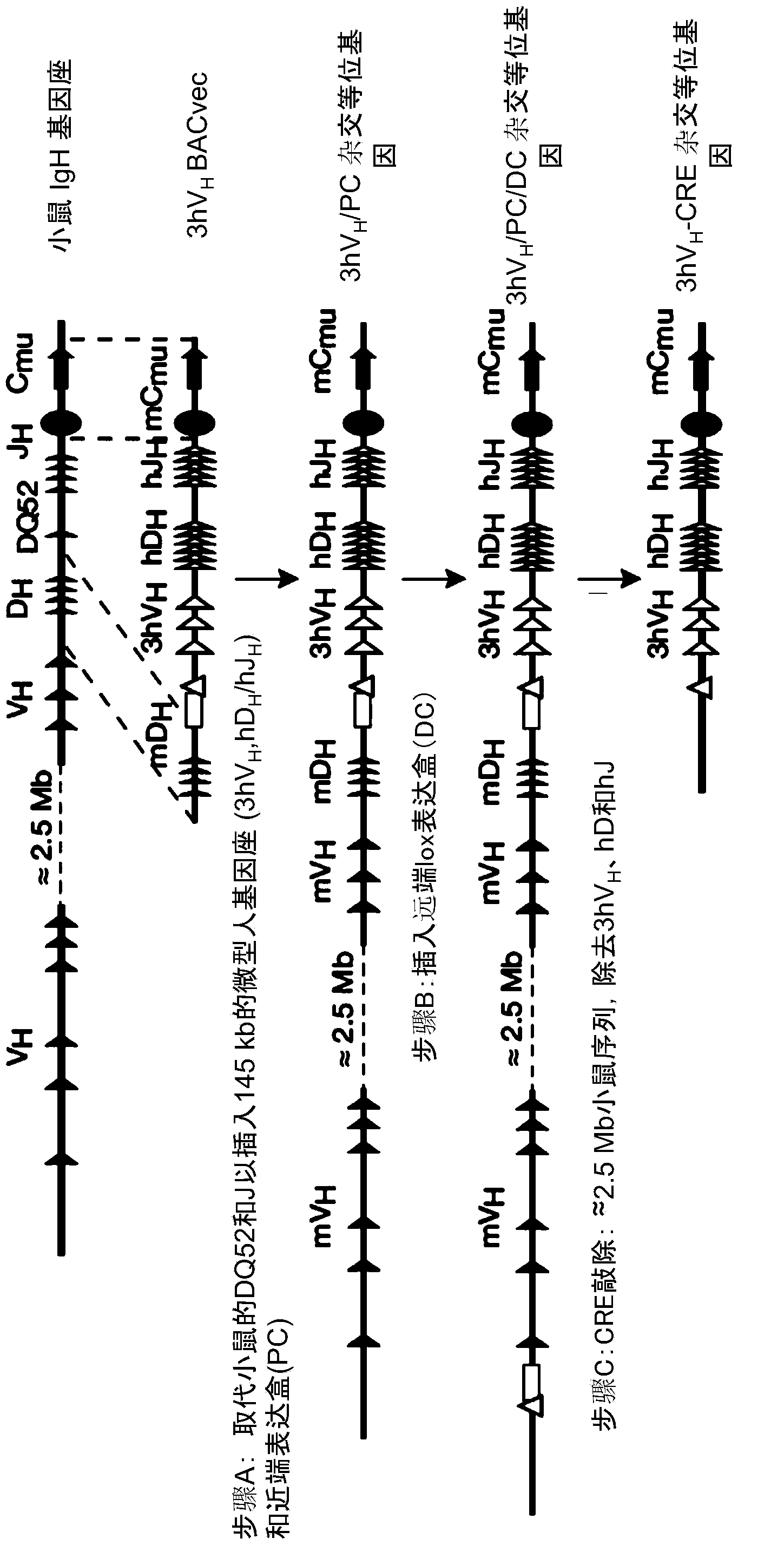
[0239] Humanization of mouse immunoglobulin genes
[0240] Thirteen different BAC targeting vectors (BACvec) were engineered using human and mouse bacterial artificial chromosomes (BACs) to humanize mouse immunoglobulin heavy chain and kappa light chain loci. Tables 1 and 2 give a detailed description of the steps performed to construct all BACvecs for humanization of the mouse immunoglobulin heavy chain and kappa light chain loci, respectively.
[0241] Identification of human and mouse BACs
[0242] Mouse BACs spanning the 5' and 3' ends of the immunoglobulin heavy chain and kappa light chain loci were identified by hybridization with filters of the BAC library or by PCR screening of the mouse BAC library DNA pool. Using a probe corresponding to the region of interest, hybridize the filter under standard conditions. The library pool is screened by PCR using unique primer pairs flanking the targeted region of interest. Additional PCRs were performed using the same primers ...
Embodiment II
[0272] Generation of fully humanized mice by combining multiple humanized immunoglobulin alleles
[0273] ES cells carrying a portion of the human immunoglobulin heavy chain or kappa light chain variable repertoire as described in Example 1 were microinjected at several locations and the resulting mice were bred to produce various types of Humanized mice with progressively increasing proportions of the human germline immunoglobulin repertoire (Table 5; Figure 5A and 5B ). 1 (V1) humanized mice have 18 human V H Gene segments and all human D H and J H gene segments, as well as 16 human Vκ gene segments and all human Jκ gene segments. 2 (V2) humanized mice and (V3) humanized mice have an increased variable lineage with a total of 39 V H and 30 Vκ, and 80 V H and 40 Vκ. Because encoding mouse V H 、D H and J H gene segments and the genomic regions of the Vκ and Jκ gene segments have been completely replaced, so any version of Antibodies produced by humanized mic...
Embodiment III
[0278] Lymphocyte populations in mice with humanized immunoglobulin genes
[0279] Three different formats were evaluated by flow cytometry Mature B cell populations in mice.
[0280] Briefly, cell suspensions from bone marrow, spleen and thymus were obtained using standard methods. According to the manufacturer's instructions, cells were plated at 5 x 10 5 cells / ml were resuspended in BD Pharmingen FACS staining buffer, blocked with anti-mouse CD16 / 32 (BD Pharmingen), stained with the appropriate antibody mix, and stained with BD CYTOFIX TM fixed. The final cell pellet was resuspended in 0.5 mL of staining buffer and stained using BD FACSCALIBUR TM and BD CELLQUEST PRO TM software for analysis. All antibodies (BD Pharmingen) were prepared in substance diluent / mix and spiked to 0.5 mg / 10 5 final concentration of cells. The antibody mixture (A-D) used for bone marrow staining is as follows: A: anti-mouse IgM b -FITC, anti-mouse IgM a -PE, anti-mouse CD45R(B220)-APC; ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com