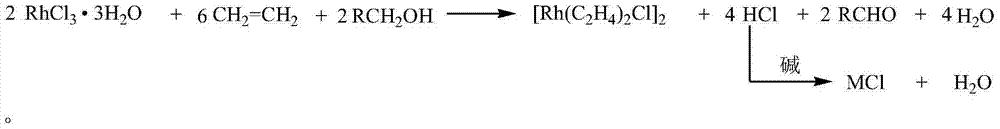
Method for synthesizing di(ethylene) chlorine rhodium (I) dimer
A dimer, hydrated rhodium trichloride technology, applied in chemical instruments and methods, compounds containing elements of group 8/9/10/18 of the periodic table, organic chemistry, etc., can solve the problem of reducing product yield and purity , Restricting large-scale production, low reaction efficiency and other problems, to achieve the effect of improving synthesis yield, improving yield and simplifying the operation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Add 20g (76mmol) rhodium trichloride hydrate into 30mL water, stir and dissolve at 50°C, after the rhodium trichloride is completely dissolved, add it to a 1L autoclave, then add 350mL of 95% ethanol, and set the autoclave at 1MPa Pressure nitrogen was replaced three times to remove the air, and the nitrogen was pressurized to 1MPa for the third time and kept for 30 minutes to check the tightness of the autoclave. After confirming the seal, replace the nitrogen with 1MPa ethylene three times, and finally adjust the ethylene pressure in the autoclave to 1MPa, start the stirring reaction, control the temperature at 30°C, and continue to feed ethylene gas when the pressure starts to decrease to keep the pressure at the set value. After continuing the reaction for 1 hour, release ethylene to bring the pressure in the autoclave to atmospheric pressure, inject 30.5 g of 20% sodium hydroxide solution with nitrogen, and continue to feed ethylene gas after sealing, keep the press...
Embodiment 2
[0025] Control reaction temperature 50 ℃, other reactant charging amount and experimental condition are the same as embodiment 1, finally obtain di(ethylene)chlororhodium (I) dimer 11.9g, yield 80.5%, have a small amount of elemental rhodium because reaction temperature is too high generate.
Embodiment 3
[0027] Maintain 2MPa ethylene pressure reaction. The feeding amount of other reactants and the experimental conditions are the same as in Example 1, and finally 12.9 g of di(ethylene)chlororhodium (I) dimer is obtained, with a yield of 87.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com