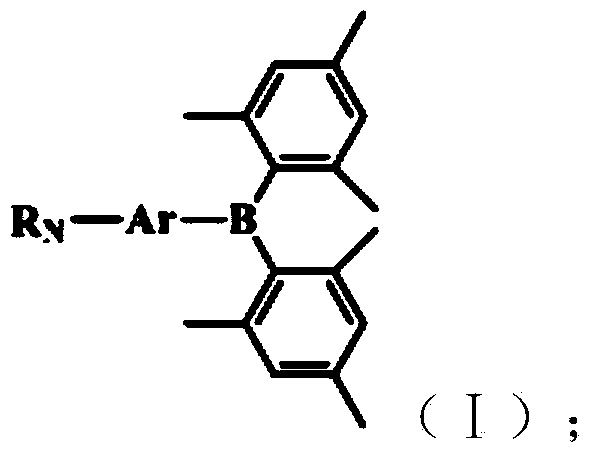
Bi(trimethylphenyl) boron derivatives and application thereof in white organic light-emitting diode
A technology of boron derivatives and trimethylbenzene, which is applied to bis(trimethylphenyl)boron derivatives and its application in white light organic electroluminescent diodes, and can solve the problem of non-luminescence mechanism and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
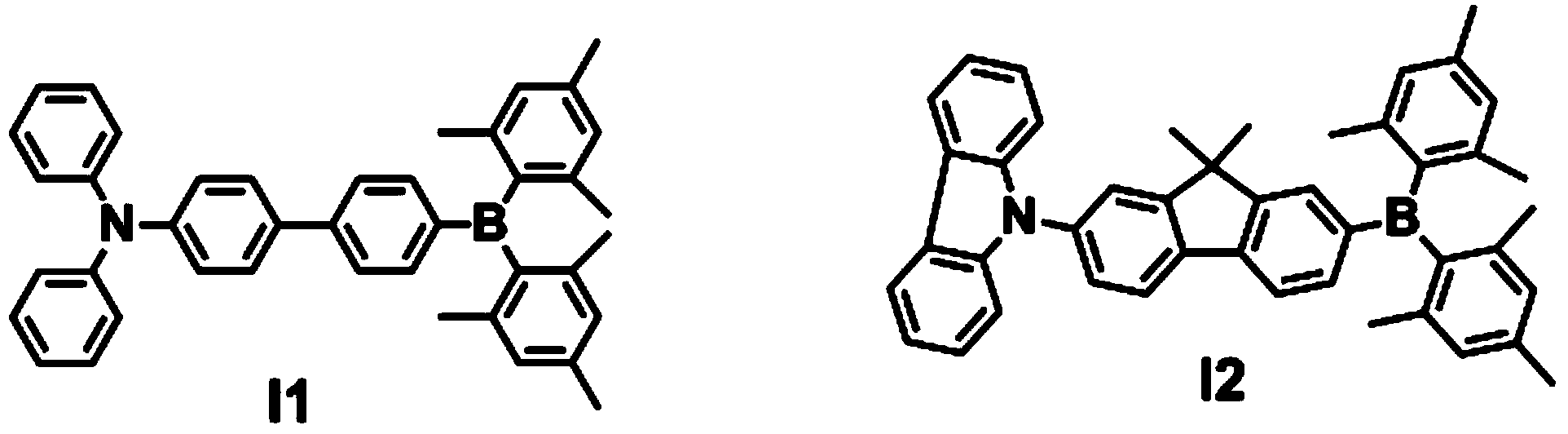
[0076] Synthesis of compound I1: the synthetic route is as follows:
[0077]
[0078] Synthesis of compound I1: Stir 1.6g of 4-bromo-4'-(diphenylamino)biphenyl and 20mL of tetrahydrofuran (analytical pure) at -78°C under nitrogen protection, slowly add 2.5ml dropwise to a concentration of 2.4mol / L The n-hexane solution of n-butyllithium was stirred for 1 h, then a solution of 1.6 g of bis(trimethylphenyl) boron fluoride dissolved in 10 ml of tetrahydrofuran (analytical grade) was added, and stirred for 12 h. The reaction solution was extracted with dichloromethane, washed with water and dried over anhydrous sodium sulfate, and evaporated to dryness under reduced pressure to obtain a crude product. The crude product was separated on a silica gel column. The eluent used was a mixture of dichloromethane and petroleum ether at a volume ratio of 1:3. After drying in a vacuum oven, 1.69 g of white solid I1 was obtained with a yield of 75%.
[0079] MS (m / e) of product I1: 569, c...
Embodiment 2
[0081] The synthetic route of compound I2 is as follows:
[0082]
[0083] Synthesis of compound I2: Stir 0.876g of 9-(7-bromo-9,9'-dimethyl-2-fluorenyl)carbazole and 10mL of tetrahydrofuran (analytical pure) at -78°C under nitrogen protection, slowly drop Add 1.25ml of n-butyllithium n-hexane solution with a concentration of 2.4mol / L, stir for 1h, then add a solution of 0.804g of bis(trimethylphenyl)boron fluoride dissolved in 5ml of tetrahydrofuran (analytical grade), and stir for 12h. The reaction solution was extracted with dichloromethane, washed with water and dried over anhydrous sodium sulfate, and evaporated to dryness under reduced pressure to obtain a crude product. The crude product was separated on a silica gel column, and the eluent used was a mixture of dichloromethane and petroleum ether at a volume ratio of 1:2. After drying in a vacuum oven, 0.8 g of white solid I2 was obtained, with a yield of 65%.
[0084] MS (m / e) of product I2: 607, corresponding to: ...
Embodiment 3
[0086] The synthetic route of compound I3 is as follows:
[0087]
[0088] Synthesis of compound I3: 1.1g of N-(7-bromo-9,9'-dimethyl-2-fluorenyl)di-tert-butyldiphenylamine and 10mL of tetrahydrofuran (analytical grade) were placed at -78°C under nitrogen protection Stir, slowly add 1.25ml of n-butyllithium n-hexane solution with a concentration of 2.4mol / L dropwise, stir for 1h, then add 0.804g of bis(trimethylphenyl)boron fluoride dissolved in 5ml of tetrahydrofuran (analytical pure) solution, Stir for 12h. The reaction solution was extracted with dichloromethane, washed with water and dried over anhydrous sodium sulfate, and evaporated to dryness under reduced pressure to obtain a crude product. The crude product was separated on a silica gel column, and the eluent used was a mixture of dichloromethane and petroleum ether at a volume ratio of 1:4. After drying in a vacuum oven, 0.95 g of white solid I3 was obtained, with a yield of 66%.
[0089] MS (m / e) of product I3:...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Maximum brightness | aaaaa | aaaaa |
| Maximum current efficiency | aaaaa | aaaaa |
| Maximum brightness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com