Novel sunitinib salts and preparing method thereof
A technology for sunitinib and nisin salt compounds, applied in the field of new sunitinib salts and their preparation, to achieve the effects of good solubility and low thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example
[0048] In order to illustrate the present invention in more detail, the following preparation examples are given, but the scope of the present invention is not limited thereto.
[0049] The analytical detection condition of the present invention is as follows:
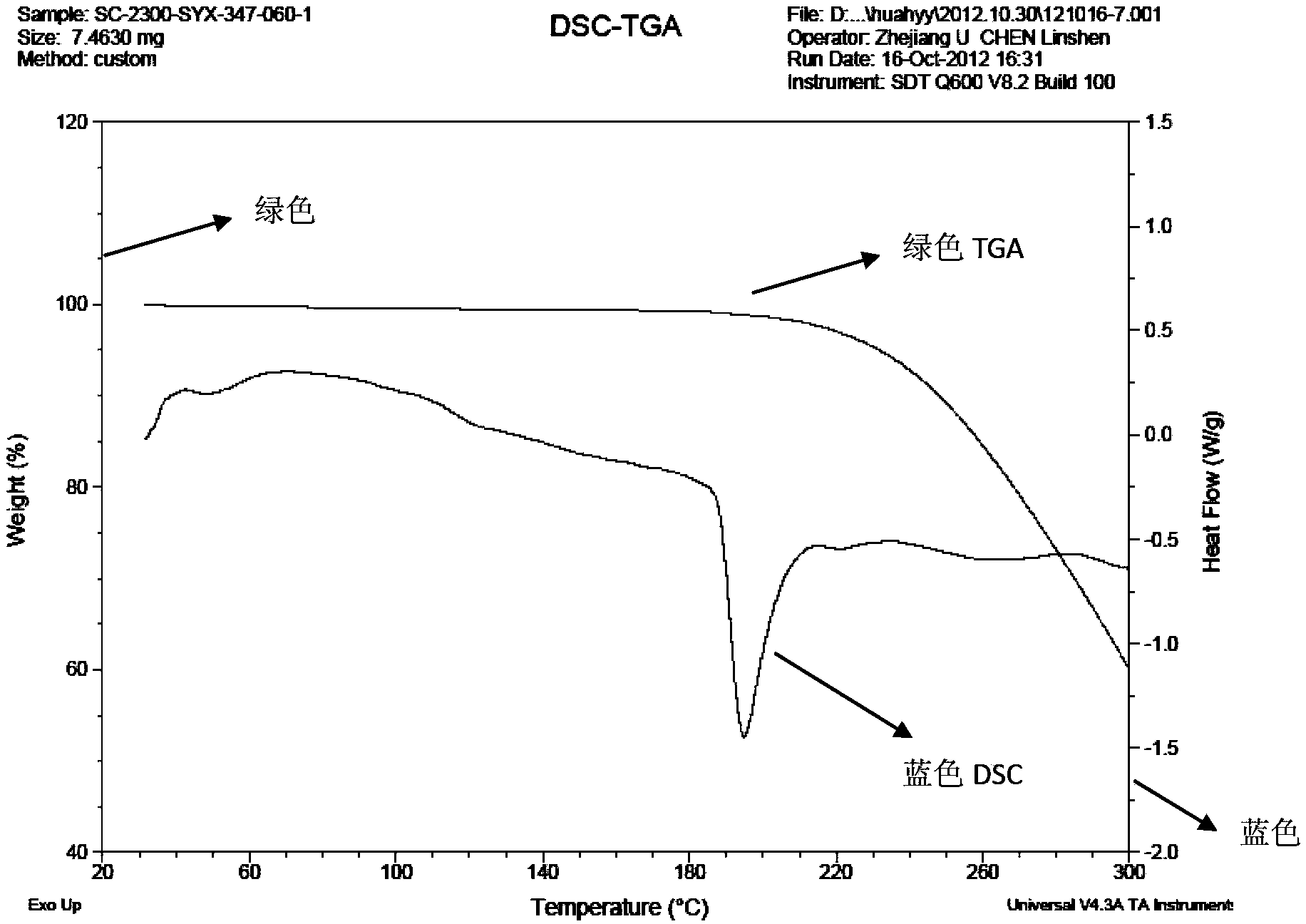
[0050] 1. DSC-TGA is measured by SDT Q600 of American TA Company, the test condition is 120ml / min N2, and the heating rate is 10℃ / min.
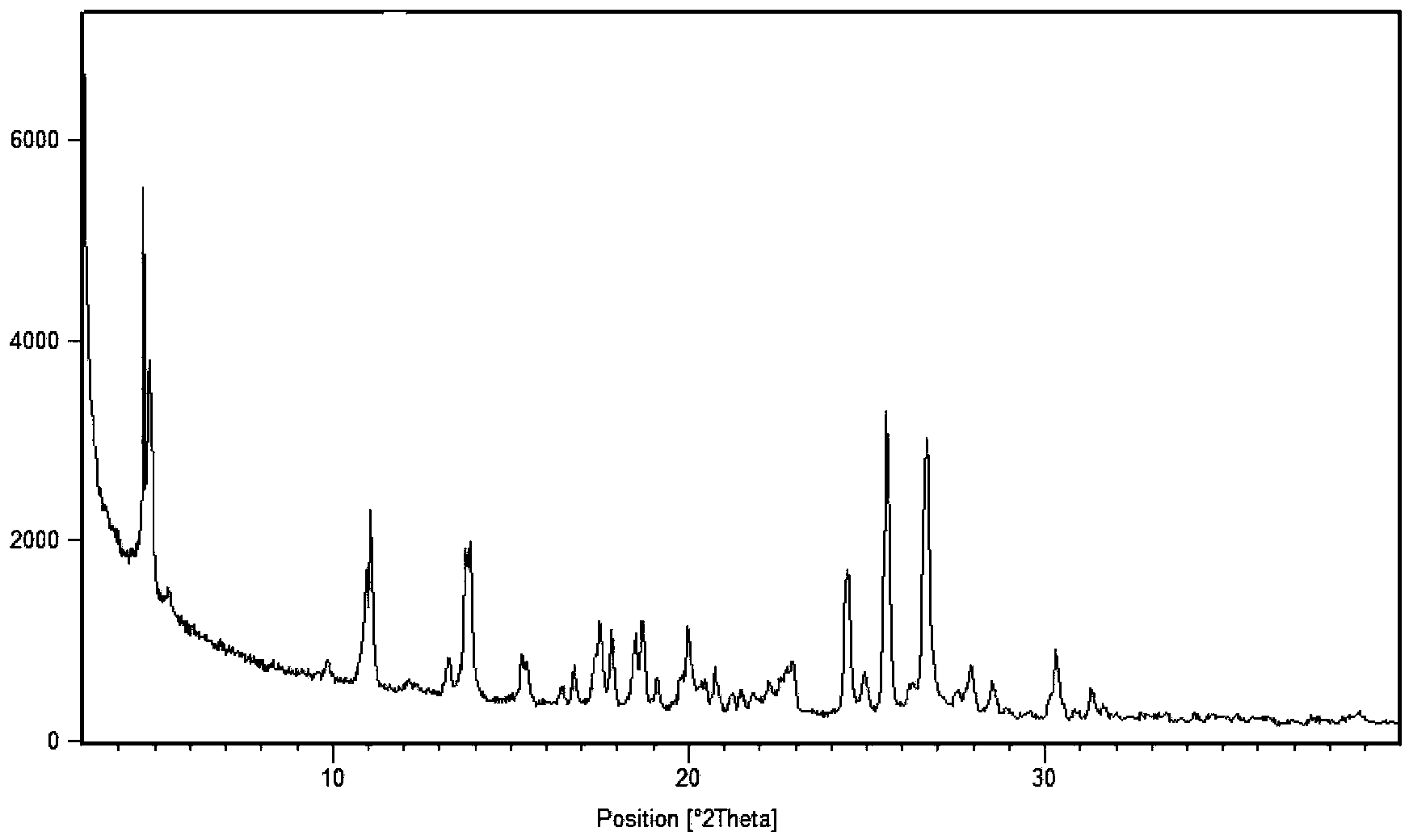
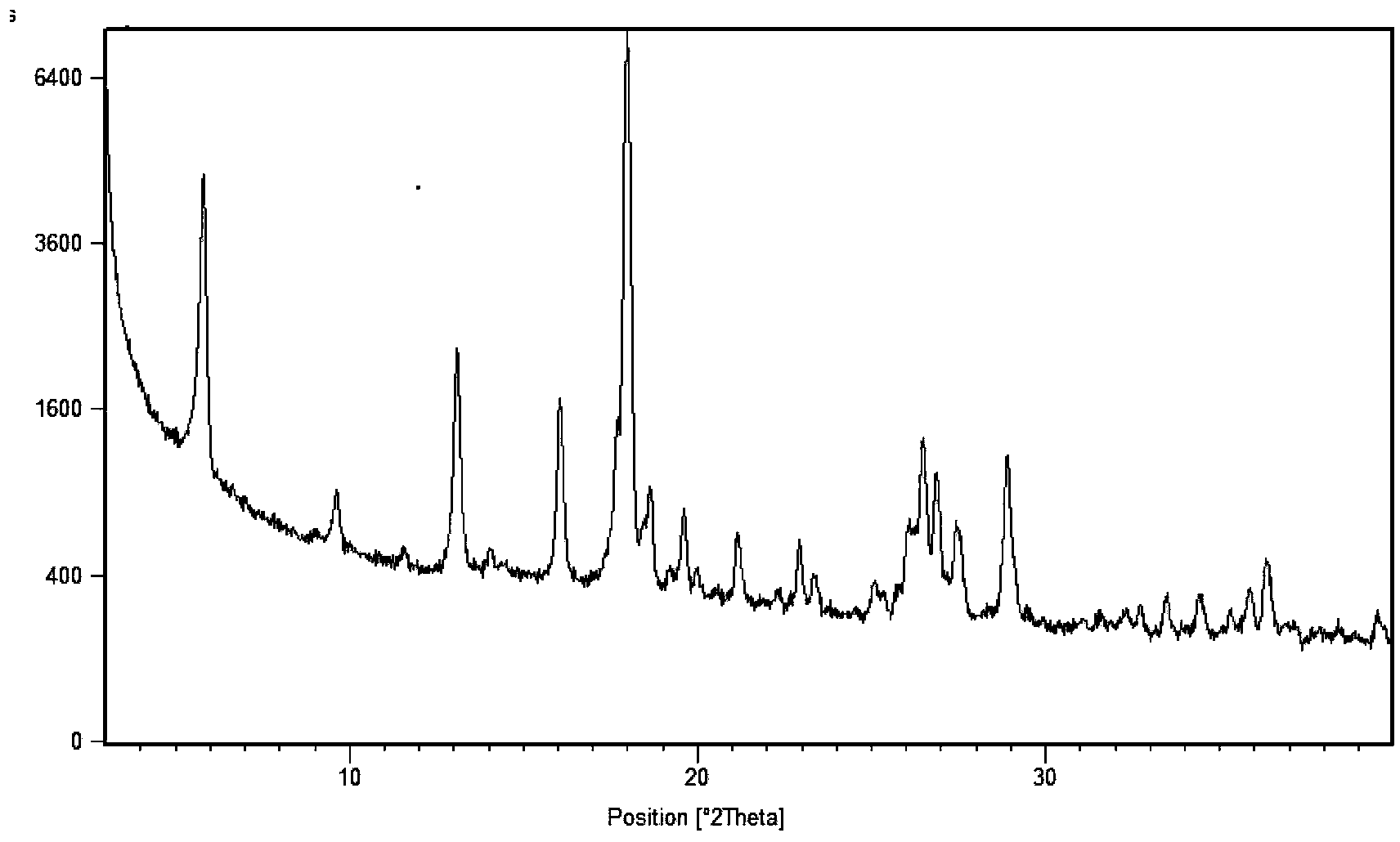
[0051] 2. X-ray powder diffraction data are measured by X′Pert Pro MPD (Multi-Purpose Diffractometer), light tube type: Empyrean XRD tube Cu LFF HR; voltage and current: 45 kV, 40 mA; goniometer: PW3050 / 60 vertical goniometer, radius 240mm; slit: DS=2°, SS=1 / 2°, mask=15mm, RS=5.0mm; detector: X′Celerator super energy detector; scanning mode: continuous scanning ; Scanning range: 3°-40°2θ; Counting time per step: 20s; Total scanning time: 6min.
Embodiment 1
[0052] Embodiment 1: sunitinib adipate
[0053] Mix 1.60 g of sunitinib and 0.60 g of adipic acid in pairs, add 60 ml of isopropanol and 6 ml of water, stir, heat to 50 ° C for 1 h to make the solution completely clear, continue to stir for 24 h, and the temperature drops to room temperature ( At about 25°C), crystals were precipitated, which were filtered by suction and washed with 10 ml of isopropanol to obtain 1.82 g of sunitinib adipate (form I).
Embodiment 2
[0054] Embodiment 2: sunitinib hemisuccinate
[0055] Mix 2.00 g of sunitinib and 0.40 g of succinic acid in pairs, add 40 ml of methanol and stir, heat to 50°C for 1 h to make the solution completely clear, then add 40 ml of ethyl acetate dropwise, continue stirring for 24 h, and the temperature drops to Crystals precipitated at room temperature (about 25°C), and were filtered with suction and washed with 10 ml of ethyl acetate to obtain 1.7 g of sunitinib hemisuccinate (form I).
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com