Dendrimer gene carrier modified by fluorine-containing aromatic ring compound as well as preparation method and application thereof
A dendrimer and gene transfection carrier technology, which is applied in the fields of polymer chemistry and biomaterials, can solve problems such as complex synthetic routes, high cytotoxicity, and high material costs, and achieve simple synthesis, high transfection efficiency, and synthetic low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
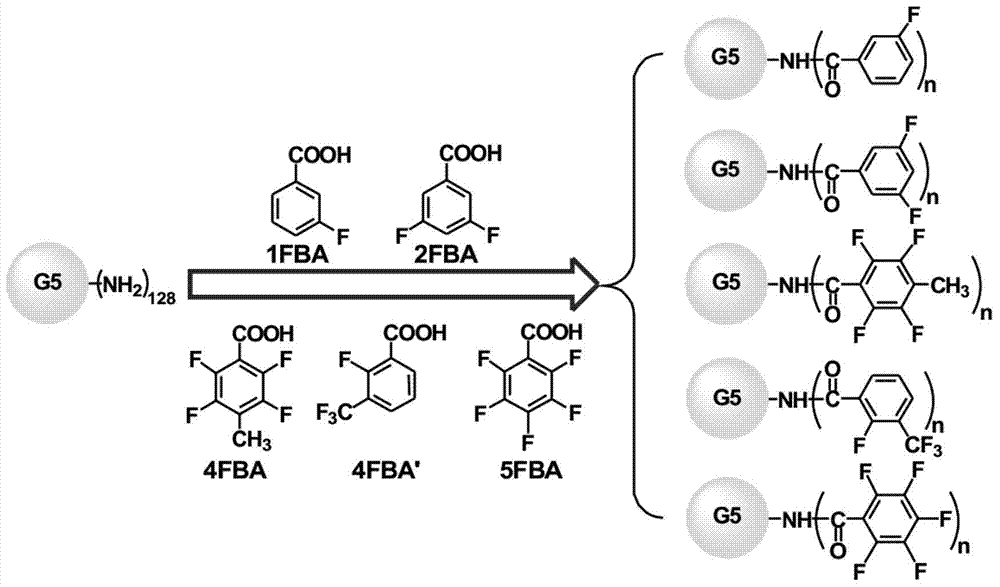
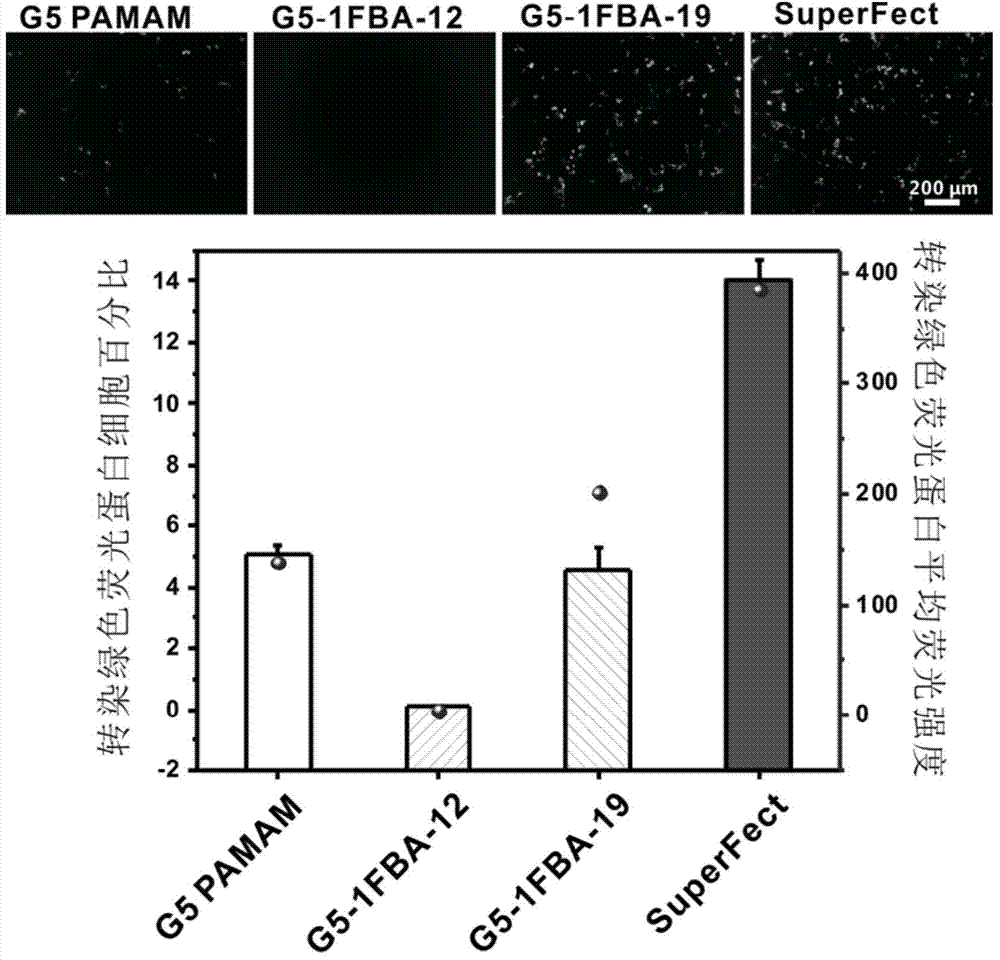
[0059] Example 1: A new material for gene transfection based on the fifth-generation polyamidoamine dendrimer modified with 3-fluorobenzoic acid was prepared at a ratio of 1:16.
[0060] Take the material based on the fifth generation polyamidoamine dendrimer modified by covalently linking 3-fluorobenzoic acid as an example, as shown in formula (1):
[0061]
[0062] Among them, the theoretical expectation of a is 16, G5 is the fifth-generation polyamide-amine dendrimer, the central core M is ethylenediamine, and the terminal group is 128 primary amine groups. Its structural formula is:
[0063]
[0064] Preparation and synthesis method: Take 3.89 mg of 3-fluorobenzoic acid, dissolve it in 3 ml of anhydrous dimethylformamide solution, add 11.57 mg of DCC, 5.75 mg of NHS, activate the reaction for 6 hours, take 50 mg of the fifth generation poly The amido-amine dendrimer was dissolved in 3 mL of anhydrous DMSO. 8.7 microliters of triethylamine was added dropwise to the abo...
Embodiment 2
[0071] Example 2: A new material for gene transfection based on the fifth-generation polyamidoamine dendrimer modified based on 3-fluorobenzoic acid was prepared at a ratio of 1:24.
[0072] Take the material based on the fifth-generation polyamidoamine dendrimer modified by covalently linking 3-fluorobenzoic acid as an example, as shown in formula (2):
[0073]
[0074] Among them, the theoretical expectation of a is 24, G5 is the fifth-generation polyamide-amine dendrimer, the central core M is ethylenediamine, and the terminal group is 128 primary amine groups. Its structural formula is:
[0075]
[0076] Preparation and synthesis method: Take 5.83 mg of 3-fluorobenzoic acid, dissolve it in 3 ml of anhydrous dimethylformamide solution, add 16.75 mg of DCC, 8.62 mg of NHS, activate the reaction for 6 hours, take 50 mg of the fifth-generation polyamide-amine The dendrimer was dissolved in 3 ml of anhydrous DMSO. 13.06 microliters of triethylamine was added dropwise to ...
Embodiment 3
[0083] Example 3: A new material for gene transfection of the fifth-generation polyamidoamine dendrimer based on 3,5-difluorobenzoic acid was prepared at a ratio of 1:16.
[0084] Take the material based on the fifth generation polyamidoamine dendrimer modified by covalently linking 3,5-difluorobenzoic acid as an example, as shown in formula (3):
[0085]
[0086] Among them, a is theoretically expected to be 16, G5 is the fifth generation polyamide-amine dendrimer, the central core M is ethylenediamine, and the terminal group is 128 primary amine groups. Its structural formula is:
[0087]
[0088] Preparation and synthesis method: Take 4.39 mg of 3,5-difluorobenzoic acid, dissolve it in 3 ml of anhydrous dimethylformamide solution, add 11.17 mg of DCC, 5.75 mg of NHS, activate the reaction for 6 hours, take 50 mg of the fifth generation poly The amido-amine dendrimer was dissolved in 3 mL of anhydrous DMSO. 8.7 microliters of triethylamine was added dropwise to the ac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com