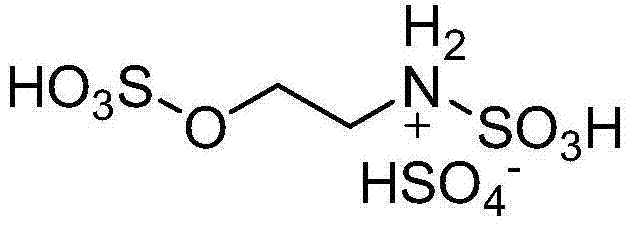
Method for preparing 2,4,5-triaryl substituted imidazole through catalysis of degradable acidic ionic liquid
A technology for the preparation of acidic ionic liquids and catalysis, applied in organic chemistry, etc., can solve the problems of large amount of ionic liquids used, difficult to biodegrade, and high preparation costs, and achieve the effect of less catalysts used, cheap raw materials, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
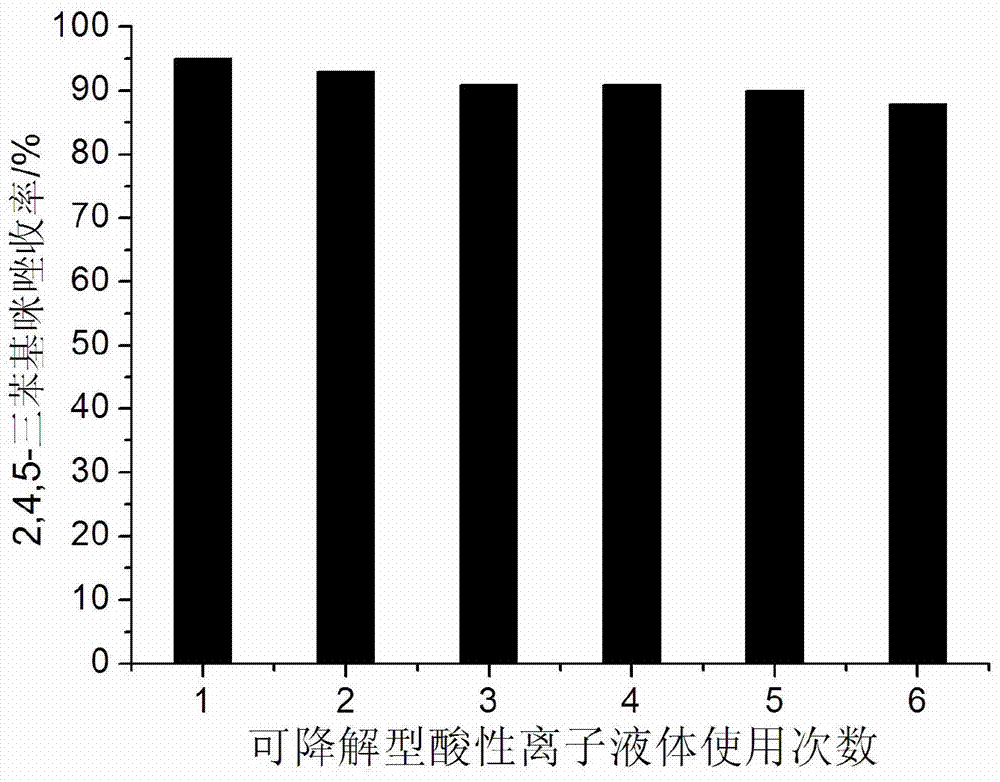
[0022] Example 1: 5mmol benzaldehyde, 5mmol benzil, 10mmol ammonium acetate and 0.15mmol degradable acidic ionic liquid were respectively added to 50ml single-necked bottles containing 5ml ethanol with a stirring bar and a condenser tube. Reflux reaction for 1.2h under vigorous stirring, TLC (thin plate chromatography, developer is V (ethyl acetate): V (petroleum ether) = 1:3) detection, the raw material point disappeared, distilled ethanol by rotary evaporator, added 15ml A large amount of solids precipitated after watering, crushed the solids, stood still, and filtered with suction. The resulting filter residue was washed with water, dried, and then recrystallized with ethanol. After vacuum drying, pure 2,4,5-triphenylimidazole was obtained with a yield of 95%. After the filtrate was evaporated to remove water, the reaction raw materials and ethanol were directly added for repeated use.
[0023] 2,4,5-Triphenylimidazole: m.p.269°C; 1 H NMR (400MHz, DMSO-d 6 ): δ=7.17~8.05(...
Embodiment 2
[0024] Example 2: 5mmol p-chlorobenzaldehyde, 5mmol benzil, 10mmol ammonium acetate and 0.20mmol degradable acidic ionic liquid were added to 50ml single-necked bottles containing 10ml ethanol with a stirring bar and a condenser. Reflux reaction for 1.5h under vigorous stirring, TLC (thin plate chromatography, developer is V (ethyl acetate): V (petroleum ether) = 1:3) detection, the raw material point disappeared, distilled ethanol by rotary evaporator, added 15ml A large amount of solids precipitated after watering, crushed the solids, stood still, filtered with suction, washed the obtained filter residue with water, dried and recrystallized with ethanol, and obtained pure 2-(4'-chlorophenyl)-4,5-diphenyl Kiimidazole, the yield is 81%. After the filtrate was evaporated to remove water, the reaction raw materials and ethanol were directly added for repeated use.
[0025] 2-(4'-chlorophenyl)-4,5-diphenylimidazole: m.p.266~268℃; 1 H NMR (400MHz, DMSO-d 6 ): δ=7.14~8.01(m, 14H...
Embodiment 3
[0026] Example 3: 5mmol o-chlorobenzaldehyde, 5mmol benzil, 10mmol ammonium acetate and 0.20mmol degradable acidic ionic liquid were added to 10ml ethanol in a 50ml single-necked bottle with a stirring bar and a condenser. Reflux reaction for 2 hours under vigorous stirring, TLC (thin plate chromatography, developer is V (ethyl acetate): V (petroleum ether) = 1:3) detection, the raw material point disappeared, distilled ethanol by rotary evaporator, added 15ml of water Afterwards, a large amount of solids precipitated, crushed the solids, stood still, and filtered with suction. The resulting filter residue was washed with water, dried, and then recrystallized with ethanol. After vacuum drying, pure 2-(2'-chlorophenyl)-4,5-diphenyl Imidazole, the yield is 78%. After the filtrate was evaporated to remove water, the reaction raw materials and ethanol were directly added for reuse.
[0027]2-(2'-chlorophenyl)-4,5-diphenylimidazole: m.p.196~198℃; 1 H NMR (400MHz, DMSO-d 6 ): δ=7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com