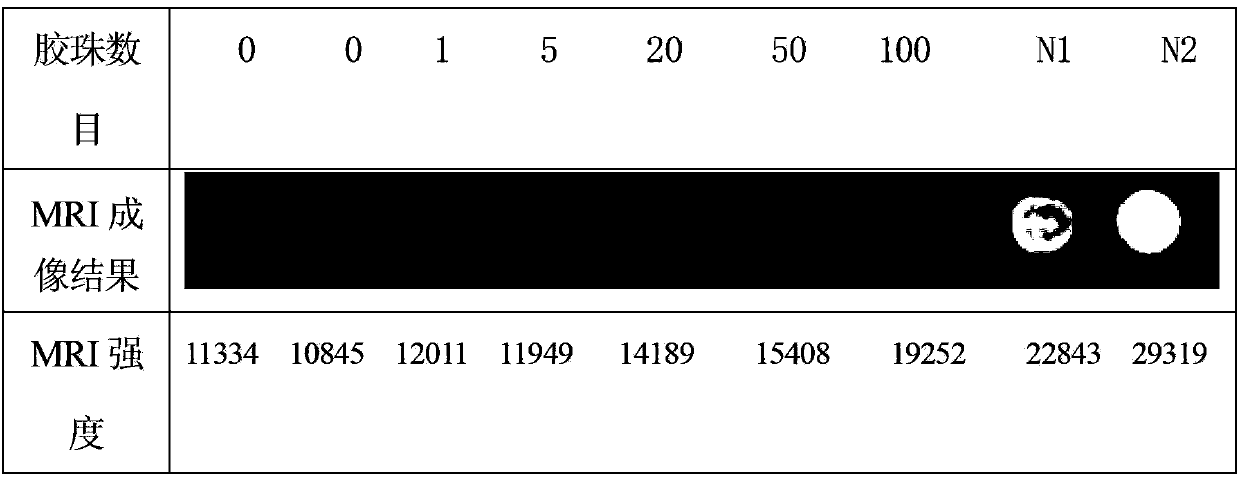
MRI development embolism microballoon based on gadolinium alginate
A technology of alginic acid and sodium alginate, applied in preparations for in vivo experiments, medical science, emulsion delivery, etc., can solve the problems of not truly reflecting the effect of embolic materials, missing the best treatment period for patients, and toxic and side effects , to achieve the effect of intuitive visual controllability, good biodegradability and good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Preparation of Gadolinium Alginate Gel Beads Containing MRI Visualizable Groups:
[0031] 1. Solution preparation
[0032] 1) 0.1mol / L GdCl 3 Solution: Take 13.18g (0.05mol) of GdCl 3 , dissolved in 500mL deionized water, after complete dissolution, filtered through a 0.22μm membrane filter, and the filtrate was collected for later use.
[0033] 2) 1.5% (w / v) sodium alginate solution: Take 1.5g of sodium alginate, dissolve it in 100mL of deionized water, stir to dissolve it completely, filter it with 0.8μm, 0.45μm, and 0.22μm filter membranes successively, and collect The filtrate was degassed overnight in a refrigerator at 4°C.
[0034] 2. Bead preparation
[0035] 1) Take the 1.5% (w / v) sodium alginate solution out of the refrigerator to return to room temperature, draw 2mL sodium alginate solution with a 5mL syringe, connect the standard 5# needle, place it at the designated position of the electrostatic droplet generator, and adjust the pump speed to 12.7mL / h. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com