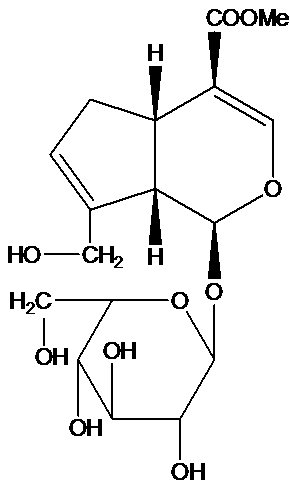
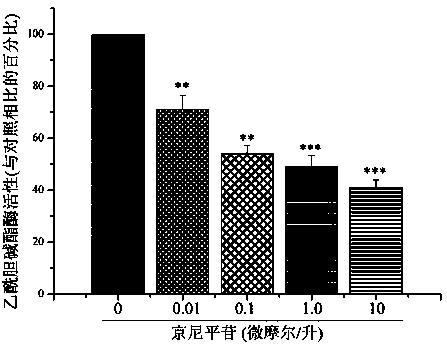
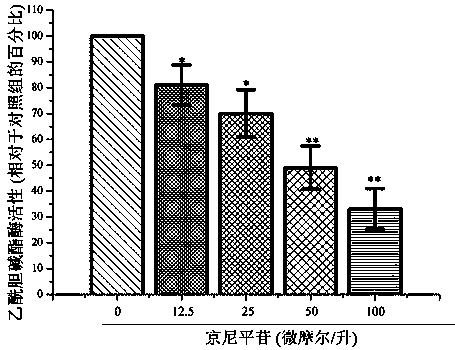
Application of geniposide used as acetylcholin esterase inhibitor
A technology of acetylcholinesterase and geniposide, applied in the application field of geniposide as an acetylcholinesterase inhibitor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Capsule preparation method: mix geniposide and sodium starch glycolate, then add sodium lauryl sulfate aqueous solution and carry out wet granulation. The wet material is then dried in a fluid bed, drying tray or other suitable dryer. The dried granules can then be milled to achieve a suitable particle size distribution prior to mixing with the other ingredients. The mixture is then filled into two hard gelatin capsule shells.
[0067] components Content per capsule
[0068] Geniposide 40 mg
[0069] Sodium Lauryl Sulfate 5 mg
[0071] Magnesium stearate 4 mg
[0072] Sodium starch glycolate 80 mg
[0073] Capsules total weight 200 mg.
Embodiment 2
[0075] Capsule preparation method: granulate geniposide and sodium starch glycolate with sodium lauryl sulfate aqueous solution, then dry the wet material in a fluidized bed, drying tray or other suitable dryer, and then grind the dried granules Grind to achieve a suitable particle size distribution before blending with the other ingredients before filling by weight into capsule shells.
[0076] components Content per capsule
[0077] Geniposide 40 mg
[0078] Sodium Lauryl Sulfate 5 mg
[0080] Talc 4 mg
[0081] Colloidal silicon dioxide 4 mg
[0082] Magnesium stearate 4 mg
[0083] Sodium starch glycolate 80 mg
[0084] Capsules total weight 200 mg.
Embodiment 3
[0086] Tablet preparation method: granulate geniposide, sodium starch glycolate and microcrystalline cellulose with an aqueous solution of sodium lauryl sulfate, and then dry the wet material in a fluidized bed, drying tray or other suitable dryer , the dried granules are then milled to achieve a suitable particle size distribution, the mixture is compressed into tablets, and these tablets are coated if necessary.
[0087] components Content per tablet
[0088] Geniposide 40 mg
[0089] Sodium Lauryl Sulfate 6 mg
[0090] Microcrystalline Cellulose 40 mg
[0091] Sodium starch glycolate 60 mg
[0093] Magnesium stearate 5 mg
[0094] Tablets total weight 200 mg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com