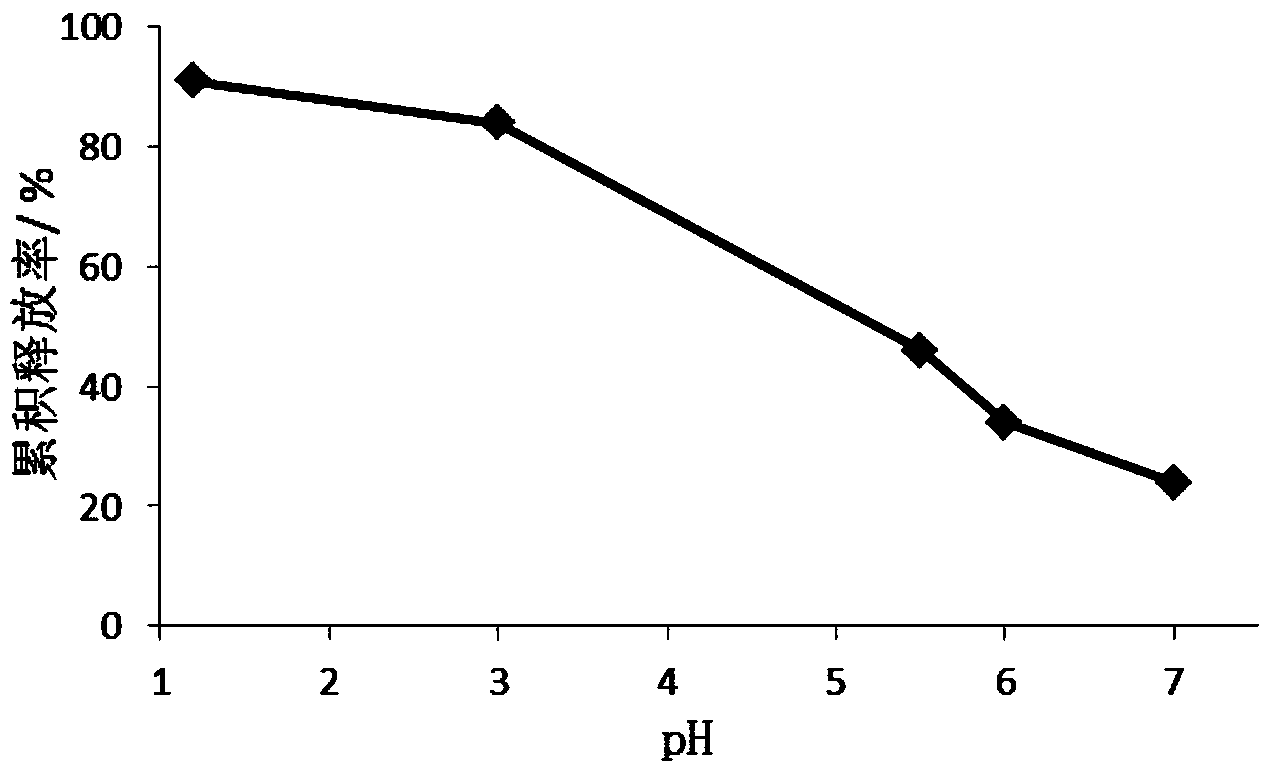
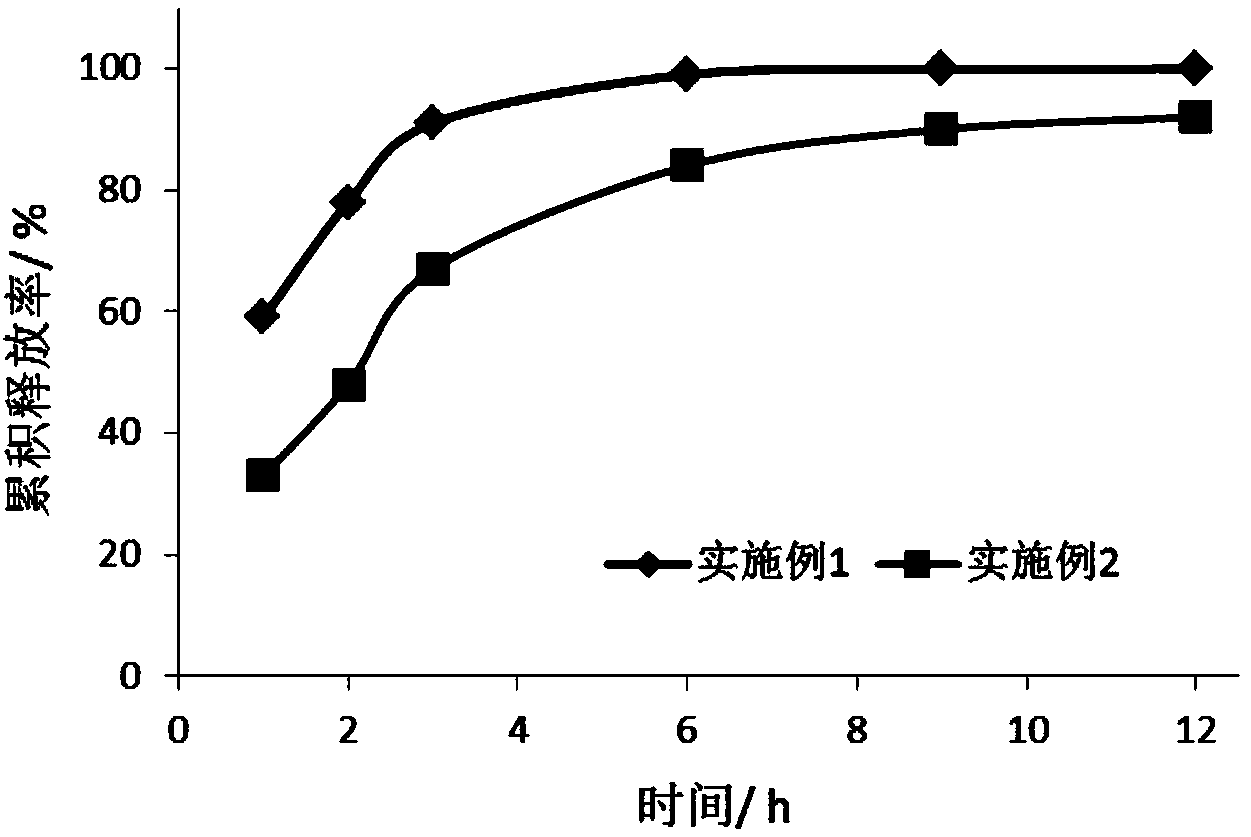
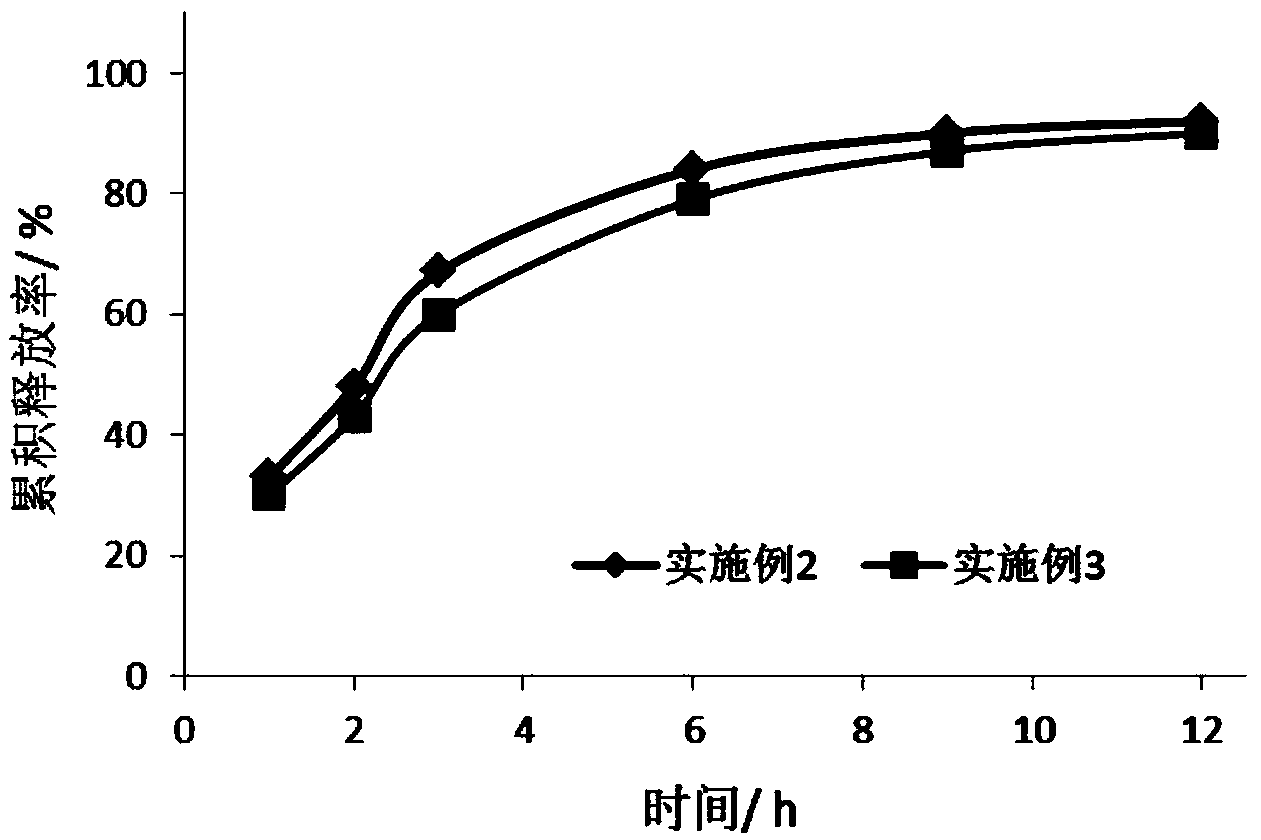
Antiallergic drug sustained release suspension and preparation method of suspension
A slow-release suspension and anti-allergic technology, which can be used in drug combinations, pharmaceutical formulas, allergic diseases, etc., and can solve problems such as uncontrollable drug release, increased energy consumption, and unfavorable quality control.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Example 1 Preparation of drug-ion exchange resin complex.
[0070] Preparation steps: (1): Dissolve 166.67g of carbinoxamine maleate in 1L of purified water; (2): Take 500g of Amberlire IRP-69 resin passed through a 80-mesh sieve, and add it to the solution obtained in (1) under stirring , continuously stirred for 4 hours, stood still, and filtered to obtain a jelly; (3): Add 2L of purified water to the jelly obtained in step (2), stir slowly for 10min, remove the supernatant, and repeat step (3) Operation 1 (4): Dry the drug-resin complex obtained in (3) at 45°C until the water content is 5%, and store it in a sealed and dry place.
Embodiment 2
[0071] Example 2 Preparation of drug-containing core.
[0072] Preparation steps: (1): Dissolve 166.67g of carbinoxamine maleate in 1L of purified water; (2): Take 500g of Amberlire IRP-69 resin passed through a 80-mesh sieve, and then add it to the obtained product in (1) under stirring In the solution, stir continuously for 4 hours, let it stand, and filter to obtain a jelly; (3): Add 2L of purified water to the jelly obtained in (2), stir slowly for 10min, remove the supernatant, and repeat step (3) Operation 1 (4): Dry the drug-resin complex obtained in (3) at 45°C until the water content is 50%; (5): 15.00g of sodium alginate passed through a 80-mesh sieve and 3.22 Add g of povidone slowly to the drug-resin complex obtained in (4) under stirring, and stir evenly; (6): Dry the mixture obtained in (5) at 45°C to a water content of 25%, and grind it with a grinder for about After 10 minutes, pass through an 80-mesh sieve, then dry at 45°C until the water content is 5%, grin...
Embodiment 3
[0073] Example 3 Preparation of drug-containing core.
[0074] Preparation steps: (1): Dissolve 166.67g of carbinoxamine maleate in 1L of purified water; (2): Mix 500.00g of Amberlire IRP-69 and 15.00g of sodium alginate resin through an 80-mesh sieve, and then Add to the solution obtained in (1) under stirring, continue to stir for 4 hours, stand still, and filter to obtain a jelly; (3): Add 2L of purified water to the jelly obtained in (2), stir slowly for 10 minutes, and remove the supernatant , repeat step (3) once; (4): dry the jelly obtained in (3) at 45°C until the water content is 50%; (5): pass 3.22g of methyl cellulose Slowly add to the drug jelly obtained in (4) under stirring, and stir evenly; (6): Dry the mixture obtained in (5) at 45°C until the water content is 25%, grind it with a grinder for about 10 minutes, and pass through 80 mesh Sieve, then dry at 45°C to a water content of 5%, grind for 10 minutes with a grinder, and pass through an 80-mesh sieve to obtai...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com