Gefitinib medicinal composition and tablet containing gefitinib medicinal composition
A technology of gefitinib and composition, applied in the field of gefitinib pharmaceutical composition, can solve the problems of preventing drug release, limitation, dosage limitation and the like
Inactive Publication Date: 2014-06-11
JIANGSU AOSAIKANG PHARMA CO LTD
View PDF4 Cites 22 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
However, generally low-viscosity HPMC is used as a film coating material, and its dosage is limited. In this patent, the dosage of HPMC in the coated tablet is about 8 mg, and the effect of inhibiting drug precipitation is very limited; while high-viscosity HPMC has slow control Release effect, excessive weight gain of the coating will prevent the release of the drug in the immediate release drug formulation
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
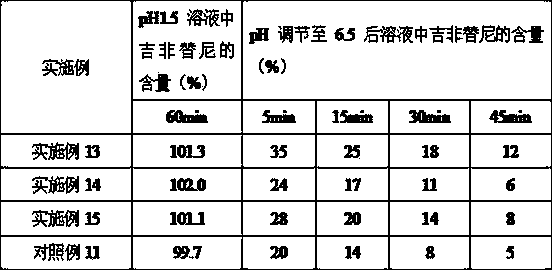
Embodiment 2
[0062] Gefitinib 250mg
[0063] Eudragit E PO 50mg
Embodiment 3
[0065] Gefitinib 250mg
[0066] Eudragit E PO 10mg
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Login to View More
Abstract
The invention belongs to the field of medicinal preparations, and provides a gefitinib medicinal composition and a tablet thereof. The gefitinib medicinal composition contains polyacrylic resin substances. By adopting the provided composition and tablet, the precipitating speed of gefitinib from a solution can be well controlled, and the bioavailability of gefitinib is improved.
Description
[0001] technical field [0002] The invention belongs to the field of pharmaceutical preparations, in particular to a gefitinib pharmaceutical composition and a tablet containing the gefitinib pharmaceutical composition. [0003] Background technique [0004] Gefitinib was first disclosed in the international patent application WO96 / 33980, and its chemical name is N-(3-chloro-4-fluorophenyl)-7-methoxy-6-(3-morpholine-4-propoxy Base) quinazoline-4-amine, the structure is shown in the following formula: [0005] . [0006] Gefitinib is a selective epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor developed by AstraZeneca. In August 2002, Japan first approved gefitinib tablets (Iressa, Iressa). ) as a drug for the treatment of non-small cell lung cancer. In February 2005, the State Food and Drug Administration approved gefitinib tablets for the treatment of locally advanced or metastatic non-small cell lung cancer who had previously received chemotherapy....
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): A61K31/5377A61K47/32A61K9/20A61K9/36A61P35/00
Inventor 陈庆财赵小伟赵俊陈玉红
Owner JIANGSU AOSAIKANG PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com