Self-assembled polypeptide, preparation method and application thereof
A self-assembly and self-selection technology, applied in the field of pharmaceutical excipients, can solve problems such as poor water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] The preparation of embodiment 1 polypeptide
[0067] Chloromethyl polystyrene resin is used as an insoluble solid-phase self-assembly. First, the amino acid whose amino group is protected by a blocking group (G) is covalently linked to the solid-phase self-assembly. Under the action of trifluoroacetic acid, the protective group of the amino group is removed, so that the first amino acid is connected to the solid phase self-assembly. Then the carboxyl group of the second amino acid whose amino group is blocked is activated by N,N'-dicyclohexylcarbodiimide (DCC, Dicyclohexylcarbodiimide), and the carboxyl group is activated by DCC. The amino group of the first assembled amino acid reacts to form a peptide bond, thus generating a dipeptide with a protecting group on solid-phase self-assembly. Repeat the above peptide bond formation reaction to grow the peptide chain from the C-terminus to the N-terminus until the desired length of the peptide chain is reached. Using th...
Embodiment 2
[0076] The complex of embodiment 2 paclitaxel and self-assembling polypeptide
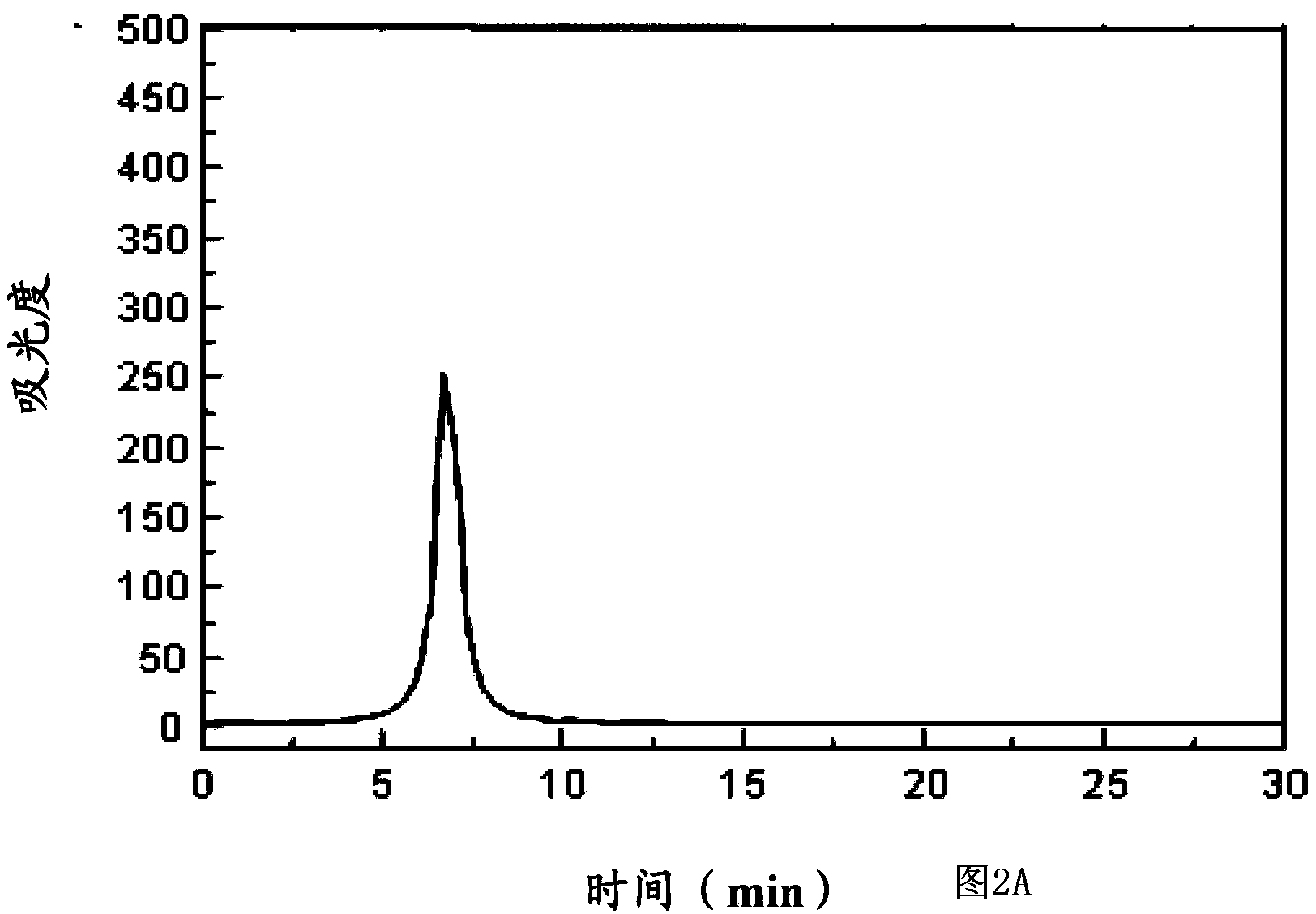
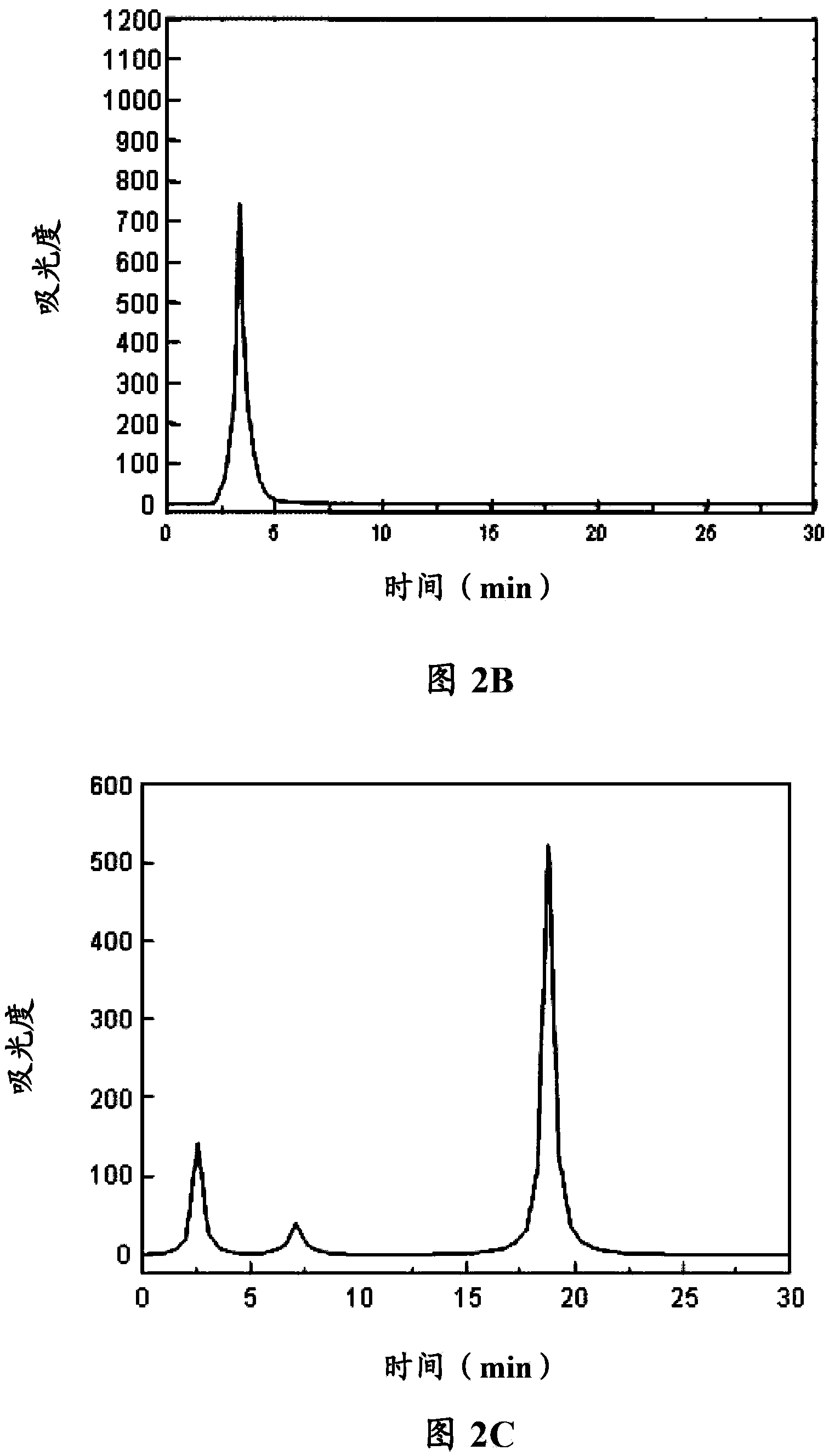
[0077] Paclitaxel (purchased from Shanghai Demo Chemical Co., Ltd.) and the self-assembled polypeptide 1 prepared in Example 1 were respectively 25:1, 1:100, 10:1, 1:25 and 1:50 (the following ratios do not indicate the same is the molar ratio), dissolved in an appropriate amount of normal saline, mixed thoroughly, and mixed ultrasonically for 5 minutes at 4°C to obtain a complex solution, and the monomer and complex were measured by HPLC method, the specific HPLC The instrument is (Agilent 1100), the mobile phase is A acetonitrile (1 / 1000 trifluoroacetic acid), mobile phase B water (1 / 1000 trifluoroacetic acid), filler octadecylsilane bonded silica gel, detection The conditions are: detection wavelength: 214nm, gradient conditions for elution: mobile phase A changes from 35 vol% to 50 vol%, and mobile phase B changes from 65 vol% to 50 vol% within 50 minutes.
[0078] Violatriol monomer see f...
Embodiment 3
[0079] The preparation of embodiment 3 pharmaceutical compositions
[0080] Put 0.05g of poloxamer, 0.2g of mannitol, 0.1g of lactose, and 3mL of water for injection into a 10mL container, stir to dissolve, add 1mol / L citric acid to adjust the pH to 6.0, cool to 5°C, Add 5 mL of the self-assembling polypeptide complex solution containing the amino acid sequence shown in SEQ ID NO: 2 prepared by the method in Example 2 into it, continue to adjust the pH to 6.0, and add water to 10 mL. Add 10mg of activated carbon, stir at 5°C for 20 minutes, remove the activated carbon by filtration, filter and sterilize with a microporous membrane (Millipore, Inc), and divide the filtrate into 0.2mL per tube, pre-freeze for 2 hours, then freeze Press and dry for 12 hours until the temperature of the sample reaches 5°C, then dry for 2 hours to obtain a white loose block, seal it to obtain the pharmaceutical composition of the complex, put it in a prefilled syringe, and the specification is 10...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Solubility | aaaaa | aaaaa |
| Solubility | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com