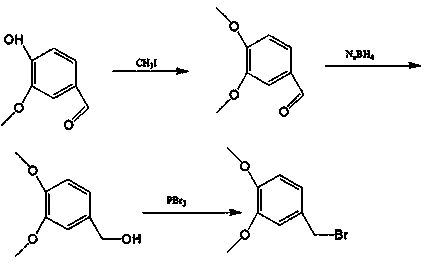
Preparation method of 3,4-dimethoxybenzyl bromide
A technology of dimethoxybenzyl and methylation, which is applied in the field of preparation of 3,4-dimethoxybenzyl bromide, can solve the problems of toxicity of triphenylphosphorus, low yield and the like, and achieves low cost, High yield and the effect of expanding production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] The preparation of embodiment 1 veratraldehyde
[0020] Weigh 20g of vanillin and 27.2-36.3g of potassium carbonate into a single-necked bottle equipped with a thermometer, stir in an ice bath for 30min, slowly add 10.6-12.3ml of methyl iodide dropwise, keep the temperature for 1-2h, stir at room temperature for 20-22h, and react When finished, cool to room temperature.
Embodiment 2
[0021] Example 2 Preparation of Veratrol
[0022] The above reaction solution was stirred in an ice bath for 30 minutes, and 6.0-7.5 g of sodium borohydride was slowly added in batches, and reacted at room temperature for 4-7 hours.
Embodiment 3
[0023] Embodiment 3 3, the preparation of 4-dimethoxybenzyl bromide
[0024] The above reaction solution was cooled to -5-0°C, 6.1-9.8ml of boron tribromide was added dropwise, and reacted at room temperature for 5-6 hours. After the reaction was completed, it was concentrated under reduced pressure, recrystallized from methanol, and the weight yield was 80-85%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com