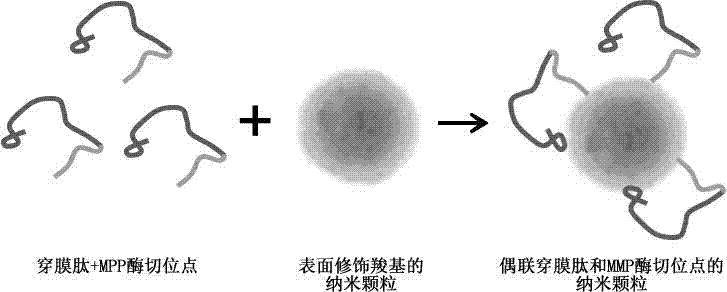
Nanoparticle coupled with coupling cell-penetrating peptide and metal matrix proteinase (MMP) restriction enzyme digestion site
A technology of nanoparticles and enzyme cleavage sites, which is used in gene carriers, drug carriers, in vivo tissue cell imaging, and tumor treatment fields. control issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] In vivo NMR imaging of magnetic nanospheres coupled with MAP-N membrane-penetrating peptide and MMP cleavage site
[0022] Solid-phase synthesis of KLKLALALALAPLGLAG peptide, which contains MAP-N penetrating peptide and MMP enzyme cleavage site sequence. By electrostatic self-assembly method, the surface of magnetic nanoparticles is sequentially modified with positively charged poly(diallyldimethylammonium chloride), PDADMAC and negatively charged polyacrylic acid (Poly(acrylic acid), PAA) to obtain carboxylated magnetic nanocomposite particles. Then, the carboxyl group on PAA was activated by 1-ethyl-3 (3-dimethylaminopropyl) carbodiimide hydrochloride (1-ethyl-3 (3-dimethylamino prowl)-carbodiimide, EDC) React with the amino group on the peptide (KLKLALALALAPLGLAG), so that the peptide is successfully connected to the surface of the magnetic particle ( figure 1 ).
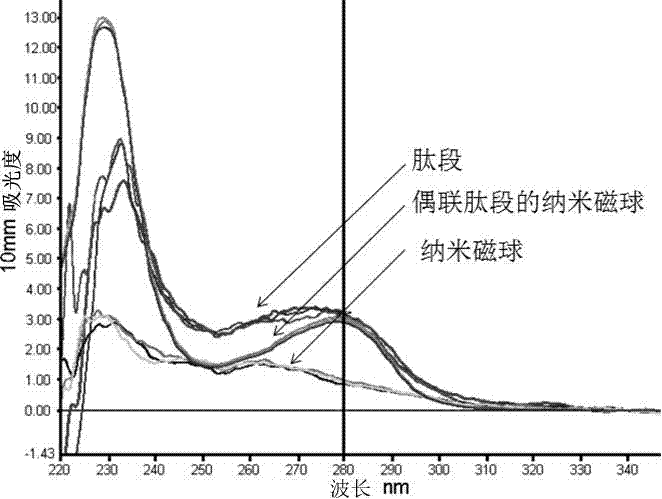
[0023] Use the UV method at a wavelength of 280nm to directly test the peptide, and select the Warb...
Embodiment 2
[0025] Example 2: In vivo magnetothermal treatment of superparamagnetic nano-magnetic spheres coupled with SNS membrane-penetrating peptide and MMP cleavage site
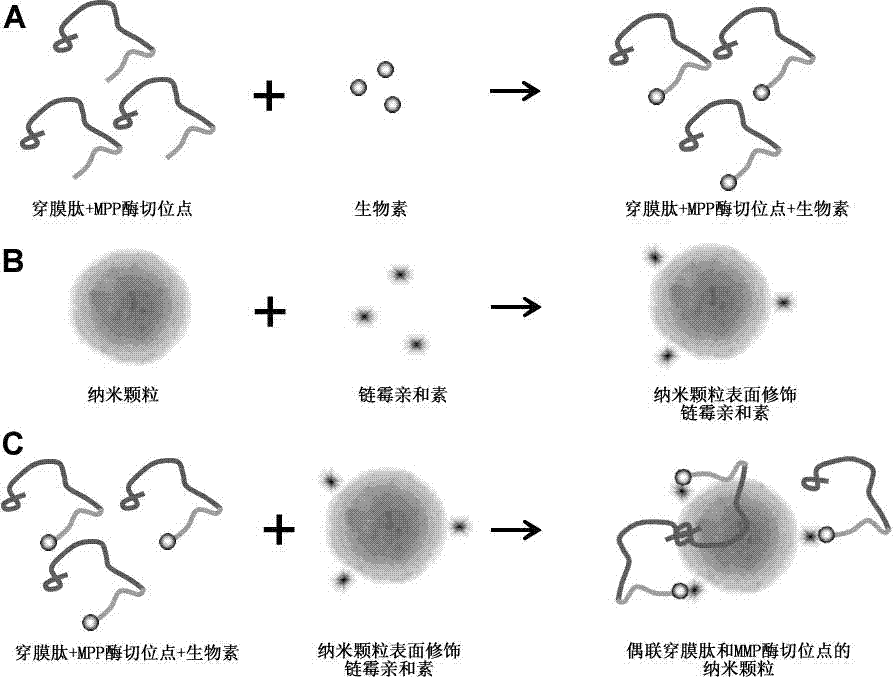
[0026] Solid-phase synthesis of AAVALLPAVLLALLAP PLGLAG peptide, which contains SNS penetrating peptide and MMP enzyme cleavage site sequence. AAVALLPAVLLALLAP PLGLAG peptide carboxyl-terminus linked to biotin. Surface modification of superparamagnetic nanospheres with streptavidin. Through the affinity reaction of biotin and streptavidin, the peptides were successfully linked to the surface of superparamagnetic nanospheres ( figure 2 ), the method for judging whether the peptide is coupled to the magnetic nanosphere is to directly test the peptide by UV method.
[0027] Inject superparamagnetic nanospheres coupled with peptides into the tail vein of the tumor-bearing animal model. After 24 hours, place the animal in an alternating magnetic field so that the internal temperature of the tumor site reaches 42°C and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com