Method for predicating acute toxicity of organophosphorus pesticide on aquatic organisms through quantitative structure activity relationship
A technology of quantitative structure-activity relationship and organophosphorus pesticides, which is applied in the direction of prediction, chemical property prediction, data processing application, etc., to achieve the effect of high-efficiency prediction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
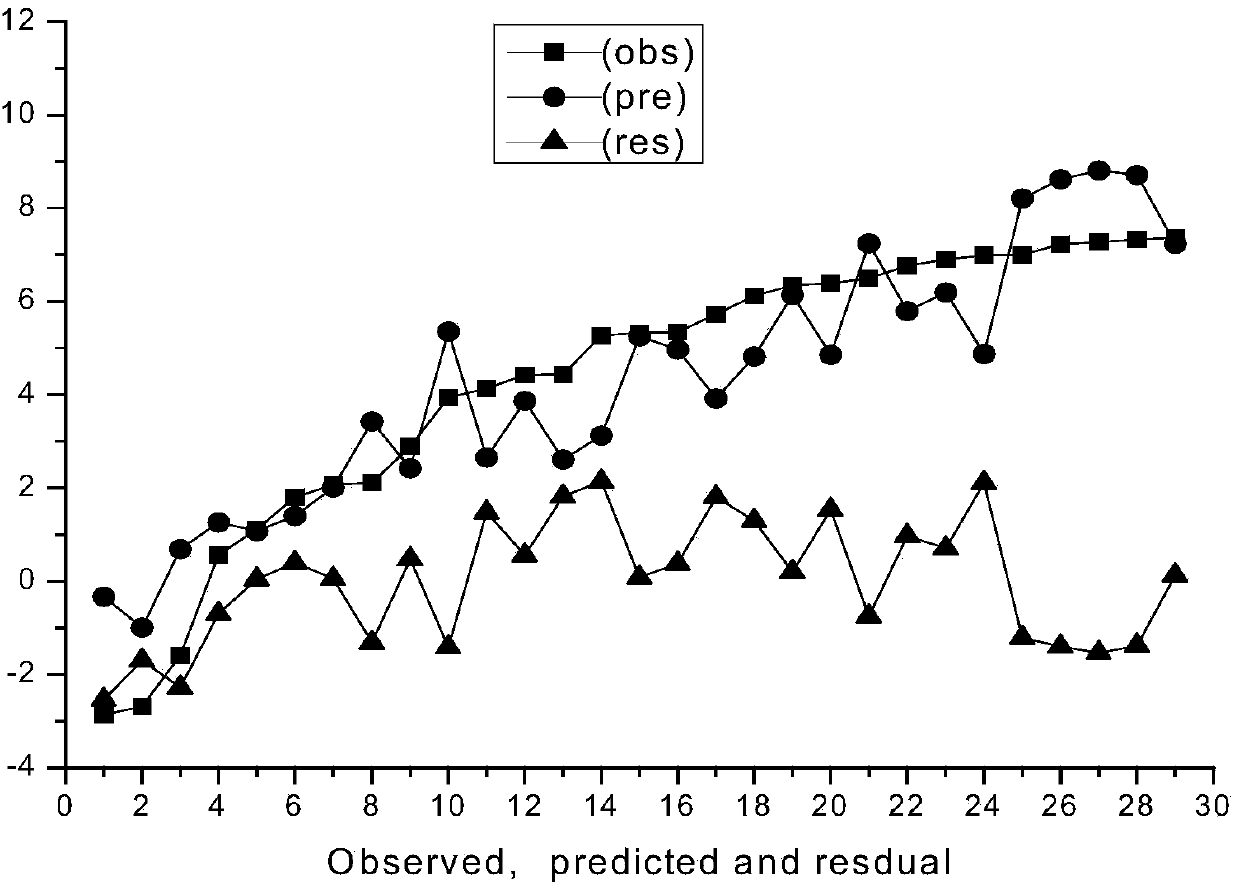
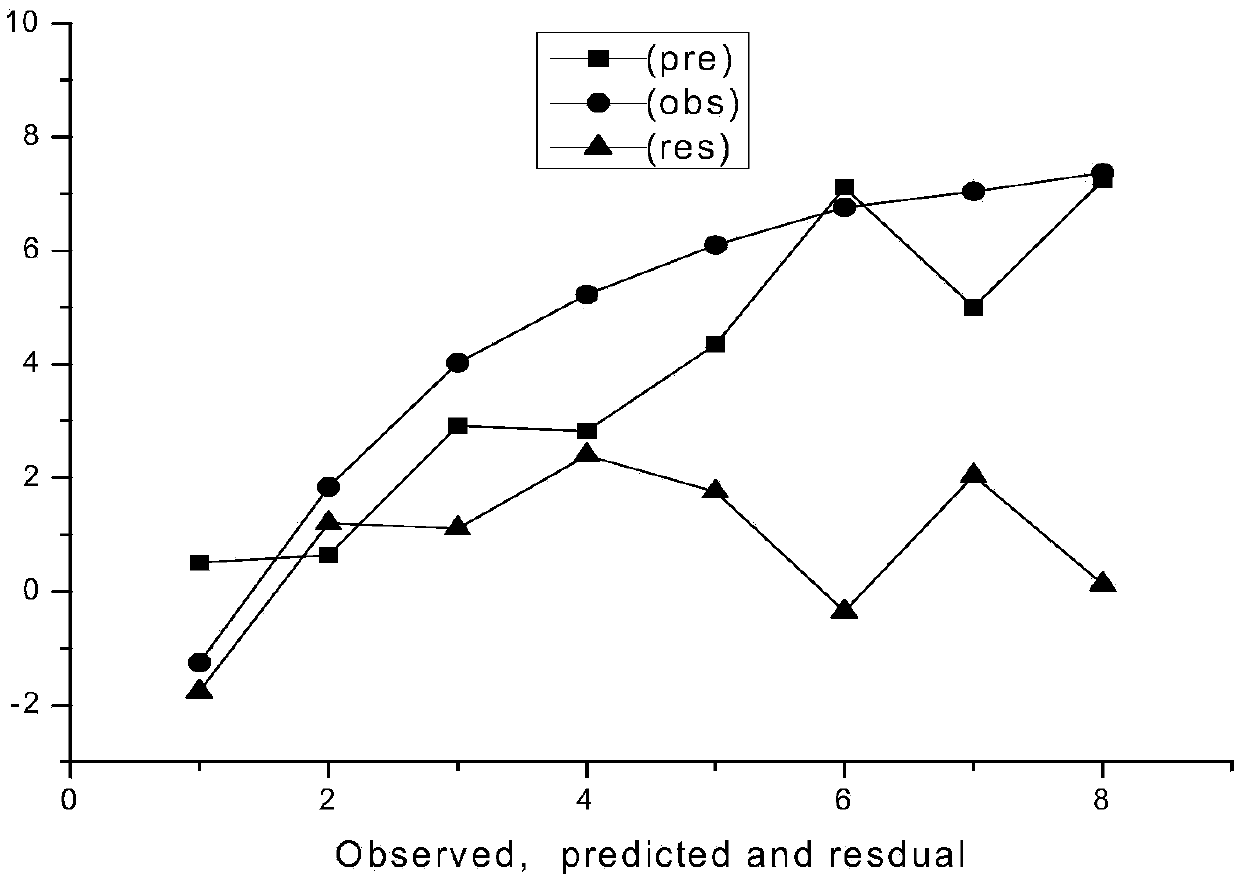
[0023] Example 1 The prediction model of the present invention is used to predict the toxicity of organophosphorus pesticide sulfonate to green algae.
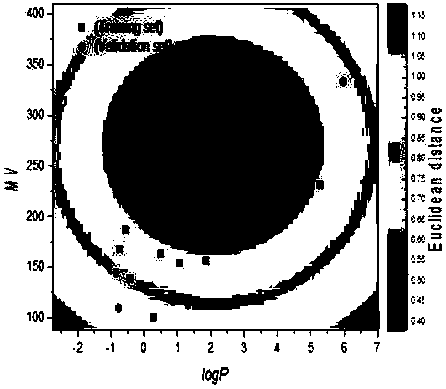
[0024] First check the molecular structure information of sulfone absorbing phosphorus, and then use Gaussian (quantum chemistry software Gaussian) to optimize the molecular structure to obtain the optimal configuration of the sulfone absorbing phosphorus structure, and then obtain the descriptor molecular volume MV required by the model and check Get its logP value. Then it was characterized by the Euclidean distance method in AmbitDiscovery (application and characterization software), and it was found that it was within the application domain of the model, so using this model to predict the toxicity of sulfone uptake phosphorus can obtain reliable results.
[0025] The predicted value of the logarithm value of its toxicity obtained at last is 7.237, while the logarithm value of the experimental value of its toxicity is 7.367...
Embodiment 2
[0026] Example 2 Application of prediction model to predict the toxicity of organophosphorus pesticide tethiophos to green algae.
[0027] Firstly, Gaussian is used to optimize the molecular structure to obtain the descriptor molecular volume MV required by the model and find its logP value. It was then found by using the Euclidean distance method to be outside the domain of application of the model, so the model could not be used to predict tethiophos toxicity.
[0028] If the model is used to predict, the predicted value of the logarithmic value of toxicity is 0.504, while the logarithmic value of the experimental value of toxicity is -1.260, with an error as high as 1.764, which is quite different from the experimental value.
Embodiment 3
[0029] Example 3 The prediction model was used to predict the toxicity of the organophosphorus pesticide methamidophos to green algae.
[0030] Firstly, Gaussian is used to optimize the molecular structure to obtain the descriptor molecular volume MV required by the model and find its logP value. It was then found to be within the domain of application of the model by using the Euclidean distance method, so the model could be used to predict methamidophos toxicity.
[0031] The predicted value of the logarithmic value of its toxicity is 7.110, while the logarithmic value of the experimental value of its toxicity is 6.761, the error is only -0.349, which is also very close to the experimental value.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com