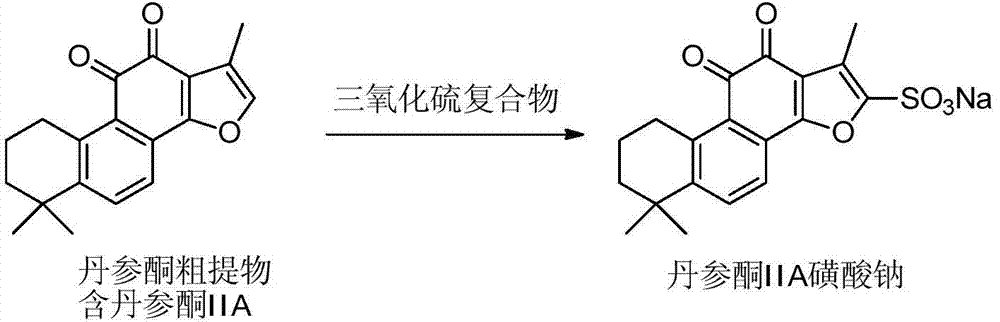
Method for preparing tanshinone IIA sodium sulfonate by using tanshinone crude extract
A technology of tanshinone and sodium sulfonate, applied in the fields of steroids, organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Add 200 g of tanshinone crude extract (tanshinone IIA content is about 60%) to 800 ml of acetonitrile solvent, then add 80 g of sulfur trioxide-pyridine complex with stirring. The reaction solution was heated to 65 degrees Celsius and stirred, and cooled to room temperature after the reaction was completed. The reaction solution was filtered, and the filter cake was washed 3-4 times with acetonitrile. Suspend the filter cake in 500 ml of hot ethanol, and add saturated ethanol solution of sodium bicarbonate until the pH value is 7.5-9.0. Remove the insoluble impurities by filtration, remove part of the ethanol solution by rotary evaporation, recrystallize after cooling, and filter to obtain the brown-red sodium tanshinone IIA sulfonate product.
Embodiment 2
[0027] Add 200 grams of crude tanshinone extract (tanshinone IIA content is about 50%) into 1000 ml of ethyl acetate solvent, and then add 100 grams of sulfur trioxide-diisopropylethylamine complex with stirring. The reaction solution was heated to reflux with stirring, and cooled to room temperature after the reaction. The reaction solution was filtered, and the filter cake was washed 3-4 times with ethyl acetate. Suspend the filter cake in 300 ml of water, add 50% aqueous sodium hydroxide solution to pH 7.5-9.0. Remove insoluble impurities by filtration, remove part of the aqueous solution by rotary evaporation, recrystallize after cooling, and filter to obtain the brown-red sodium tanshinone IIA sulfonate product.
Embodiment 3
[0029] Add 200 grams of tanshinone crude extract (tanshinone IIA content is about 80%) to 1000 ml of dichloroethane solvent, and then add 120 grams of sulfur trioxide-pyridine complex with stirring. The reaction solution was heated to 140 degrees Celsius and stirred, and cooled to room temperature after the reaction was completed. The reaction solution was filtered, and the filter cake was washed 3-4 times with dichloroethane. Suspend the filter cake in 600 ml of acetone, and add saturated aqueous sodium carbonate solution until the pH value is 7.5-9.0. Remove insoluble impurities by filtration, remove part of the acetone solution by rotary evaporation, recrystallize after cooling, and filter to obtain the brown-red sodium tanshinone IIA sulfonate product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com